
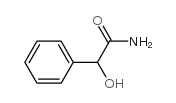
2-羟基-2-苯基乙酰胺结构式

|
常用名 | 2-羟基-2-苯基乙酰胺 | 英文名 | (+/-)-Mandelamide |
|---|---|---|---|---|
| CAS号 | 4410-31-5 | 分子量 | 151.16300 | |
| 密度 | 1.246 g/cm3 | 沸点 | 345.5ºC at 760 mmHg | |
| 分子式 | C8H9NO2 | 熔点 | 133-137ºC(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 162.7ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
The mandelamide keto-enol system in aqueous solution. Generation of the enol by hydration of phenylcarbamoylcarbene.
J. Am. Chem. Soc. 125(1) , 187-94, (2003) Flash photolysis of diazophenylacetamide in aqueous solution produced phenylcarbamoylcarbene, whose hydration generated a transient species that was identified as the enol isomer of mandelamide. This assignment is based on product identification and the shape... |
|
|
[Molecular biology aspects of the antimicrobial effect of synthetic chemotherapeutic agents].
Z. Arztl. Fortbild. (Jena.) 76(15) , 662-6, (1982)
|
|
|
Catalytic asymmetric addition of dimethylzinc to alpha-ketoesters, using mandelamides as ligands.
Org. Lett. 8(7) , 1287-90, (2006) [reaction: see text] A strategy based on the control of the electron-donating capabilities of the coordinating groups of the ligand has been applied in the catalytic asymmetric addition of organometallic reagents to ketoesters. Mandelamides having deprotonate... |
|
|
Synthesis and fungicidal activity of N-2-(3-methoxy-4-propargyloxy)phenethyl amides. Part II: anti-oomycetic mandelamides.
Pest Manag. Sci. 62(5) , 446-51, (2006) Novel types of anti-oomycetic compounds have been designed and prepared. The synthetic approach to these mandelamides is outlined. Biological data demonstrate their high efficacy against important plant diseases such as tomato and potato late blight (Phytopht... |
|
|
Synthesis and fungicidal activity of N-2-(3-methoxy-4-propargyloxy) phenethyl amides. Part 3: stretched and heterocyclic mandelamide oomyceticides.
Pest Manag. Sci. 63(1) , 57-62, (2007) Novel analogues of mandipropamid have been designed and prepared. The synthetic approach to these stretched and heterocyclic mandelamides is outlined. Biological data demonstrate their high efficacy against important plant diseases like tomato and potato late... |
|
|
Identification of amino acid residues responsible for the enantioselectivity and amide formation capacity of the Arylacetonitrilase from Pseudomonas fluorescens EBC191.
Appl. Environ. Microbiol. 75(17) , 5592-9, (2009) The nitrilase from Pseudomonas fluorescens EBC191 converted (R,S)-mandelonitrile with a low enantioselectivity to (R)-mandelic acid and (S)-mandeloamide in a ratio of about 4:1. In contrast, the same substrate was hydrolyzed by the homologous nitrilase from A... |
|
|
Identification and characterization of a mandelamide hydrolase and an NAD(P)+-dependent benzaldehyde dehydrogenase from Pseudomonas putida ATCC 12633.
J. Bacteriol. 185(8) , 2451-6, (2003) The enzymes of the mandelate metabolic pathway permit Pseudomonas putida ATCC 12633 to utilize either or both enantiomers of mandelate as the sole carbon source. The genes encoding the mandelate pathway were found to lie on a single 10.5-kb restriction fragme... |
|
|
Anisotropic and hydrogen bonding effects in phenylglyoxamides and mandelamides: theoretical and NMR conformational evaluation.
Magn. Reson. Chem. 46(5) , 418-26, (2008) Interesting anisotropic effects were observed for phenylglyoxamides and their respective mandelamides. Such effects were observed in experimental (1)H and (13)C NMR (in CDCl(3), CD(3)OD, and DMSO-d(6) solvents) and in some cases with good correlation to theor... |
|
|
Using directed evolution to probe the substrate specificity of mandelamide hydrolase.
Protein Eng. Des. Sel. 22(2) , 103-10, (2009) Mandelamide hydrolase (MAH), a member of the amidase signature family, catalyzes the hydrolysis of mandelamide to mandelate and ammonia. X-ray structures of several members of this family, but not that of MAH, have been reported. These reveal nearly superimpo... |