喹啉黄
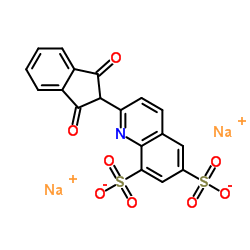
喹啉黄结构式

|
常用名 | 喹啉黄 | 英文名 | Quinoline Yellow |
|---|---|---|---|---|
| CAS号 | 8004-92-0 | 分子量 | 477.375 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C18H9NNa2O8S2 | 熔点 | 150ºC (Decomposes) | |
| MSDS | 中文版 美版 | 闪点 | N/A | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Pattern of intake of food additives associated with hyperactivity in Irish children and teenagers.
Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 27(4) , 447-56, (2010) A double-blind randomized intervention study has previously shown that a significant relationship exists between the consumption of various mixes of seven target additives by children and the onset of hyperactive behaviour. The present study set out to ascert... |
|
|
Separation of synthetic food colourants in the mixed micellar system. Application to pharmaceutical analysis.
J. Chromatogr. A. 1081(1) , 42-7, (2005) The paper presents a rapid method for the determination of commonly used synthetic food dyes by micellar electrokinetic capillary chromatography. Detection and separation conditions allowing complete resolution of 15 synthetic food colourants were investigate... |
|
|
Effects of different food colorants and polishing techniques on color stability of provisional prosthetic materials.
Dent. Mater. J. 29(2) , 167-76, (2010) The main objective was to investigate the effects of different polishing techniques on the color stability of provisional prosthetic materials upon exposure to different staining agents by mimicking the oral environment in vitro. Fifty-six cylindrical specime... |
|
|
Evaluation of certain food additives and contaminants.
World Health Organ. Tech. Rep. Ser. (966) , 1-136, (2011) This report represents the conclusions of a Joint FAO/WHO Expert Committee convened to evaluate the safety of various food additives, with a view to recommending acceptable daily intakes (ADIs) and to preparing specifications for identity and purity. The Comm... |
|
|
Liquid chromatographic determination of 2-(2-quinolinyl)-1H-indene-1,3-[2H]-dione and other organic-soluble matter in D&C Yellow No. 10.
J. Assoc. Off. Anal. Chem. 68(3) , 477-9, (1985) A sensitive, reproducible method that uses an Extrelut QE column and liquid chromatography (LC) in the reverse phase mode is described for the determination of 2-(2-quinolinyl)-1H-indene-1,3-[2H]-dione and other organic-soluble matter found in D&C Yellow No. ... |
|
|
Sensitization potentials of D & C Yellow No. 10 and D & C Yellow No. 11 dyes.
J. Am. Acad. Dermatol. 9(4) , 605-6, (1983)
|
|
|
Contact allergic reaction to D & C Yellow No. 11 and Quinoline Yellow.
Contact Dermatitis 9(4) , 263-8, (1983) D & C Yellow No. 11 and Quinoline Yellow belong to a group of quinophtalone dyes with a common basic structure. D & C Yellow No. 11 is used mostly in plastics, spirit lacquers, coloured smokes and cosmetics, but it is also increasingly used as a dye in soaps ... |
|
|
Human maximization testing of D&C Yellow no. 10 and Yellow no. 11.
Contact Dermatitis 11(4) , 210-3, (1984) By the maximization test, using 0.5% D&C Yellow no. 11 in pet., 15 of 20 human volunteers became contact sensitized. All reacted to a challenge concentration of 1000 ppm and one down to 1 ppm. The high allergenic potential of this dyestuff was confirmed. Ther... |
|
|
D&C nos. 10 and 11: chemical composition analysis and delayed contact hypersensitivity testing in the guinea pig.
Contact Dermatitis 10(1) , 30-8, (1984) D&C Yellow no. 11 was found to consist predominantly of quinophthalone together with other minor components, though not 6'-methylquinophthalone. Quinophthalone was found to be a strong sensitizer in guinea pigs by means of a modified testing technique, and it... |
|
|
Dose response relationships in delayed hypersensitivity to quinoline dyes.
Contact Dermatitis 9(4) , 309-12, (1983) Repeat insult patch testing of the quinoline dyes, D & C Yellow No. 10 (Acid Yellow No. 3) and D & C Yellow No. 11 (Solvent Yellow No. 33) demonstrated that concentrations as high as 1,000 ppm of the former induced no delayed contact hypersensitivity, whereas... |