N(e)-Boc-L-赖氨酸
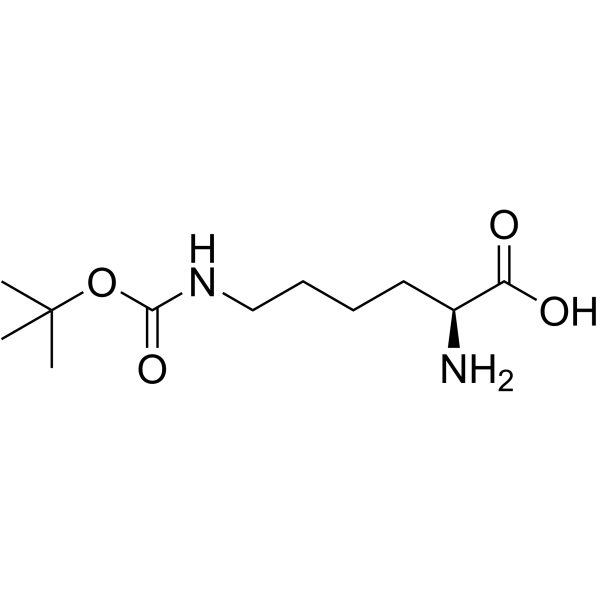
N(e)-Boc-L-赖氨酸结构式

|
常用名 | N(e)-Boc-L-赖氨酸 | 英文名 | Ne-Boc-L-lysine |
|---|---|---|---|---|
| CAS号 | 2418-95-3 | 分子量 | 246.303 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 412.9±40.0 °C at 760 mmHg | |
| 分子式 | C11H22N2O4 | 熔点 | 250 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 203.5±27.3 °C |
|
Adding l-lysine derivatives to the genetic code of mammalian cells with engineered pyrrolysyl-tRNA synthetases.
Biochem. Biophys. Res. Commun. 371(4) , 818-22, (2008) We report a method for site-specifically incorporating l-lysine derivatives into proteins in mammalian cells, based on the expression of the pyrrolysyl-tRNA synthetase (PylRS)-tRNA(Pyl) pair from Methanosarcina mazei. Different types of external promoters wer... |
|
|
Evidence that malondialdehyde-derived aminoenimine is not a fluorescent age pigment.
Chem. Res. Toxicol. 14(5) , 473-5, (2001) It has been suggested that protein modifications by malondialdehyde (MDA), a major product of lipid peroxidation, contribute to the fluorescence formation of lipofuscin. Although early studies proposed an aminoenimine structure (RNHCH=CHCH=NR) formed from MDA... |
|
|
Nanoassembly of surfactants with interfacial drug-interactive motifs as tailor-designed drug carriers.
Mol. Pharm. 10(1) , 187-98, (2013) PEGylated lipopeptide surfactants carrying drug-interactive motifs specific for a peptide-nitroxide antioxidant, JP4-039, were designed and constructed to facilitate the solubilization of this drug candidate as micelles and emulsion nanoparticles. A simple sc... |
|
|
Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose.
Biochem. J. 344 Pt 1 , 109-16, (1999) The glycation of proteins by glucose has been linked to the development of diabetic complications and other diseases. Early glycation is thought to involve the reaction of glucose with N-terminal and lysyl side chain amino groups to form Schiff's base and fru... |
|
|
Chain-length dependence for secondary structure formation of homo-oligopeptides from epsilon-tert.-butyloxycarbonyl-L-lysine with a lipophilic C-terminal group.
Int. J. Pept. Protein Res. 23(1) , 47-54, (1984) A solid-state and solution analysis of the homo-oligopeptides from epsilon-tert.-butyloxycarbonyl-L-lysine with p-oxymethylbenzylcholestan-3 beta-yl succinate as C-terminal group, using infrared absorption and circular dichroism, is described. The occurrence ... |
|
|
Well-defined homopolypeptides, copolypeptides, and hybrids of poly(l-proline).
Biomacromolecules 12 , 2396-2406, (2011) l-Proline is the only, out of 20 essential, amino acid that contains a cyclized substituted α-amino group (is formally an imino acid), which restricts its conformational shape. The synthesis of well-defined homo- and copolymers of l-proline has been plagued e... |
|
|
Synthesis of a new amphiphilic glycodendrimer with antiviral functionality.
Carbohydr. Polym. 90(2) , 1061-8, (2012) A new third generation amphiphilic glycodendrimer was synthesized from a stearylamide lysine dendrimer by condensation of the oligosaccharide moiety. By stepwise condensation and deprotection of di-boc lysine from a core of stearyl amide lysine, a third-gener... |
|
|
Isolation and characterization of a new advanced glycation endproduct of dehydroascorbic acid and lysine.
Biochim. Biophys. Acta 1620(1-3) , 235-44, (2003) Proteins are subject of posttranslational modification by sugars and their degradation products in vivo. The process is often referred as glycation. L-Dehydroascorbic acid (DHA), an oxidation product of L-ascorbic acid (vitamin C), is known as a potent glycat... |
|
|
Development of a mechanism-based assay for tissue transglutaminase--results of a high-throughput screen and discovery of inhibitors.
Anal. Biochem. 338(2) , 237-44, (2005) Tissue transglutaminase (TGase) is a Ca(2+)-dependent enzyme that catalyzes cross-linking of intracellular proteins through a mechanism that involves isopeptide bond formation between Gln and Lys residues. In addition to its transamidation activity, TGase can... |
|
|
Large-pore polydimethylacrylamide resin for solid-phase peptide synthesis: applications in Fmoc chemistry.
Pept. Res. 9(6) , 297-304, (1996) We have synthesized a hydrophilic crosslinked aminoalkyl polydimethylacrylamide-beaded support upon which peptides have been assembled using standard Fmoc chemistry in automated batch-wise equipment. The resin was prepared by the free radical-initiated co-pol... |