
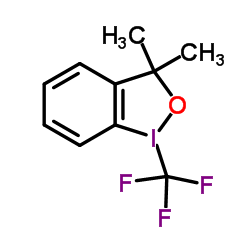
3,3-二甲基-1-(三氟甲基)-1,2-苯并碘氧杂戊环结构式

|
常用名 | 3,3-二甲基-1-(三氟甲基)-1,2-苯并碘氧杂戊环 | 英文名 | Togni's Reagent |
|---|---|---|---|---|
| CAS号 | 887144-97-0 | 分子量 | 330.086 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C10H10F3IO | 熔点 | 75-79ºC | |
| MSDS | 中文版 美版 | 闪点 | N/A | |
| 符号 |


GHS02, GHS07 |
信号词 | Warning |
|
A Ritter-type reaction: direct electrophilic trifluoromethylation at nitrogen atoms using hypervalent iodine reagents.
Angew. Chem. Int. Ed. Engl. 5th ed., 50 , 1059-1063, (2011)
|
|
|
The productive merger of iodonium salts and organocatalysis: a non-photolytic approach to the enantioselective alpha-trifluoromethylation of aldehydes.
J. Am. Chem. Soc. 132 , 4986-4987, (2010) An enantioselective organocatalytic alpha-trifluoromethylation of aldehydes has been accomplished using a commercially available, electrophilic trifluoromethyl source. The merging of Lewis acid and organocatalysis provides a new strategy for the enantioselect... |
|
|
Pd(II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter.
J. Am. Chem. Soc. 11th ed., 132 , 3648-3649, (2010) A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction. |
|
|
Copper-catalyzed trifluoromethylation of aryl and vinyl boronic acids with an electrophilic trifluoromethylating reagent.
Org. Lett. 13 , 2342-2345, (2011) A copper-catalyzed trifluoromethylation of aryl- and alkenylboronic acids with Togni's reagent was described. The reaction proceeded in good to excellent yields for a range of different substrates including heteroarylboronic acids and substrates with a variet... |
|
|
Highly selective trifluoromethylation of 1,3-disubstituted arenes through iridium-catalyzed arene borylation.
Angew. Chem. Int. Ed. Engl. 2nd ed., 51 , 540-543, (2012) The old one two: A sequential iridium-catalyzed borylation and copper-catalyzed trifluoromethylation of arenes is described (see scheme; Pin = pinacol). The reaction is conducted under mild reaction conditions and tolerates a variety of functional groups. The... |
|
|
Direct electrophilic N-trifluoromethylation of azoles by a hypervalent iodine reagent.
Angew. Chem. Int. Ed. Engl. 26th ed., 51 , 6511-6515, (2012) Effective CF(3) transfer: Various electron-rich nitrogen heterocycles (pyrazoles, triazoles, and tetrazoles) can be directly N-trifluoromethylated by a hypervalent iodine reagent in an efficient manner. The optimized procedure, which includes an in situ silyl... |
|
|
Mild electrophilic trifluoromethylation of secondary and primary aryl- and alkylphosphines using hypervalent iodine(iii)-CF(3) reagents.
Chem. Commun. (Camb.) 13 , 1575-1577, (2008) A direct, mild and efficient trifluoromethylation of primary and secondary phosphines is achieved with easily accessible, cheap hypervalent iodine compounds acting as electrophilic CF(3)-transfer reagents. |
|
|
Reactivity of a 10-I-3 Hypervalent Iodine Trifluoromethylation Reagent With Phenols Stanek, K.; Koller, R.; Togni, A.
J. Org. Chem. 73th ed., 19 , 7678-7685, (2008)
|
|
|
Electrophilic S-trifluoromethylation of cysteine side chains in a- and ß-peptides: isolation of trifluoromethylated Sandostatin (octreotide) derivatives Capone, S.; et al.
Helv. Chim. Acta 11th ed., 91 , 2035-2056, (2008)
|
|
|
Electrophilic trifluoromethylation of arenes and N-heteroarenes using hypervalent iodine reagents Wiehn, M. S.; Vinogradova, E. V.; Togni, A.
J. Fluor. Chem. 9th ed., 131 , 951-957, (2010)
|