烟酰胺1,N6-乙烯基腺嘌呤二核苷酸
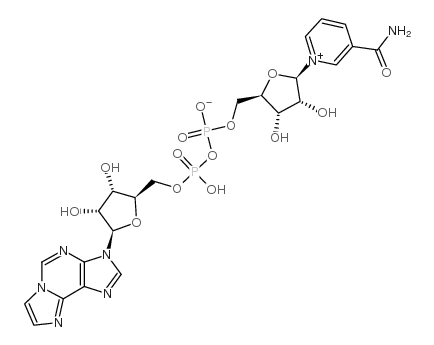
烟酰胺1,N6-乙烯基腺嘌呤二核苷酸结构式

|
常用名 | 烟酰胺1,N6-乙烯基腺嘌呤二核苷酸 | 英文名 | Nicotinamide 1,N6-Ethenoadenine Dinucleotide |
|---|---|---|---|---|
| CAS号 | 38806-38-1 | 分子量 | 687.44700 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C23H27N7O14P2 | 熔点 | N/A | |
| MSDS | 美版 | 闪点 | N/A | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Hormonal control of ADP-ribosyl cyclase activity in pancreatic acinar cells from rats.
J. Biol. Chem. 278(36) , 33629-36, (2003) Cyclic ADP-ribose, a metabolite of NAD+ evokes Ca2+ release from intracellular stores in different cells. We have determined the activity of cADPr-producing enzymes (ADP-ribosyl cyclases) in different cellular fractions prepared from isolated pancreatic acina... |
|
|
NAD+ analogs substituted in the purine base as substrates for poly(ADP-ribosyl) transferase.
FEBS Lett. 397 , 17-21, (1996) Poly(ADP-ribosyl) transferase (pADPRT) catalyzes the transfer of the ADP-ribose moiety from NAD+ onto proteins as well as onto protein-bound ADP-ribose. As a result, protein-bound polymers of ADP-ribose are formed. pADPRT itself contains several acceptor site... |
|
|
Assay for ADP-ribosyl cyclase by reverse-phase high-performance liquid chromatography.
Anal. Biochem. 299(2) , 218-26, (2001) Cyclic ADP-ribose (cADPR), a natural metabolite of beta-NAD(+), is a second messenger for Ca(2+) signaling in T cells. As a tool for purification and identification of ADP-ribosyl cyclase(s) in T cells, a sensitive and specific enzymatic assay using 1,N(6)-et... |
|
|
Age-related effects in coenzyme binding patterns of rat muscle glyceraldehyde-3-phosphate dehydrogenase.
Biochemistry 20(21) , 6041-6, (1981) The binding of NAD+ and of its fluorescent analogue, nicotinamide 1,N6-ethenoadenine dinucleotide, to glyceraldehyde-3-phosphate dehydrogenase purified from the muscles of young and old rats was studied in detail. Binding of the natural coenzyme was followed ... |
|
|
Spectrophotometric assay of NADase-catalyzed reactions.
Anal. Biochem. 116(2) , 374-8, (1981)
|
|
|
Phosphorescence maxima and triplet state lifetimes of NAD+ and epsilon-NAD+ in ternary complexes with horse liver alcohol dehydrogenase.
Photochem. Photobiol. 49(2) , 137-43, (1989) Origin and fate of pancreatic stellate cells (PSCs) before, during and after pancreatic injury are a matter of debate. The crucial role of PSCs in the pathogenesis of pancreatic fibrosis is generally accepted. However, the turnover of the cells remains obscur... |
|
|
Steady-state kinetics and transient studies of substrate and coenzyme analogue binding to clostridial glutamate dehydrogenase (GDH) during oxidative deamination.
Biochem. Soc. Trans. 22(3) , 319S, (1994)
|
|
|
Self-inactivation of an erythrocyte NAD glycohydrolase.
Mol. Cell Biochem. 31(1) , 49-56, (1980) NAD glycohydrolase activity was studied using bovine erythrocytes, erythrocyte ghosts and partially purified enzyme preparations. During catalysis the enzyme becomes irreversibly inactivated in a process related to substrate turnover. Self-inactivation was ob... |
|
|
In situ staining for poly(ADP-ribose) polymerase activity using an NAD analogue.
J. Histochem. Cytochem. 46(11) , 1279-89, (1998) Poly(ADP-ribose) polymerase (PARP) is a highly abundant nuclear enzyme which metabolizes NAD, in response to DNA strand breakage, to produce chains of poly(ADP-ribose) attached to nuclear proteins. PARP activation has been implicated in ischemia/reperfusion i... |
|
|
Fluorescence studies of ternary complexes of liver alcohol dehydrogenase.
Adv. Exp. Med. Biol. 328 , 513-21, (1993)
|