右旋达尔丰
一般危化品
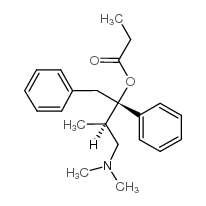
右旋达尔丰结构式
|
常用名 | 右旋达尔丰 | 英文名 | dextropropoxyphene |
|---|---|---|---|---|
| CAS号 | 469-62-5 | 分子量 | 339.47100 | |
| 密度 | 1.038 g/cm3 | 沸点 | 444ºC at 760 mmHg | |
| 分子式 | C22H29NO2 | 熔点 | 165±3ºC | |
| MSDS | N/A | 闪点 | 130.6ºC | |
| 符号 |


GHS02, GHS07 |
信号词 | Danger |
|
Clinical toxicology in Edinburgh, two centuries of progress.
Clin. Toxicol. (Phila.) 51(6) , 509-14, (2013) The Scottish Poisons Information Bureau was established in Edinburgh in September 1963 and shortly afterwards one of the wards of the city's Royal Infirmary was designated a Regional Poisoning Treatment Centre. Both units were soon to be brought under one roo... |
|
|
Diplopia with dextropropoxyphene withdrawal.
Gen. Hosp. Psychiatry 35(1) , 100-1, (2013) As an infrequent symptom diplopia has been reported with opiate withdrawal, especially heroin, but not dextropropoxyphene. We report incomitant esotropia and diplopia in a case with dextropropoxyphene withdrawal that resolved completely with the resolution of... |
|
|
Urine drug testing of chronic pain patients. V. Prevalence of propoxyphene following its withdrawal from the United States market.
J. Anal. Toxicol. 37(1) , 1-4, (2013) Propoxyphene is an opioid analgesic that was surrounded by controversy concerning its safety and efficacy during its lifespan in the US market. Propoxyphene was withdrawn in November of 2010 from the US market and is still being detected one year post-withdra... |
|
|
Physician perspective on propoxyphene as a potentially inappropriate medication in Tennessee.
South. Med. J. 104(7) , 533-9, (2011) Medicare Part D data from the Quality Improvement Organization's 9th Statement of Work drug safety indicator project under the direction of the Centers for Medicare & Medicaid Services define the potentially inappropriate medications (PIMs) list for Tennessee... |
|
|
[Which analgesic after dextropropoxyphene withdrawal? A survey in a sample of general practitioners in southwest of France].
Therapie. 66(1) , 25-8, (2011) Following the announcement of the future withdrawal in Europe of drugs containing dextropropoxyphene-paracetamol (DXP-P), we performed a postal survey in a randomly selected sample of 350 general practitioners (GP) from the Midi-Pyrénées area (2.6 million inh... |
|
|
Synthetic opioids and arrhythmia risk: a new paradigm?
Expert Opin. Pharmacother. 13(13) , 1825-7, (2012) The most commonly prescribed class of medications in the United States for chronic severe pain is opioid analgesics. Due to their low cost and widespread availability, the synthetic opioids are popular choices among clinicians and patients. However, there is ... |
|
|
Suicides involving co-proxamol fell dramatically after withdrawal in UK.
BMJ 344 , e3255, (2012)
|
|
|
Opioids, QTc prolongation, and torsades.
J. Clin. Pharmacol. 52(10) , 1614, (2012)
|
|
|
Clinical toxicology and drug regulation: a United Kingdom perspective.
Clin. Toxicol. (Phila.) 49(6) , 452-6, (2011) The Medicines and Healthcare products Regulatory Authority (MHRA) is the government body with responsibility for regulating new and existing medicines and medical devices in the United Kingdom. The Yellow Card scheme is a well-established pharmacovigilance sy... |
|
|
Propoxyphene-induced torsades de pointes.
Heart Rhythm 8(12) , 1952-4, (2011)
|