卢非酰胺
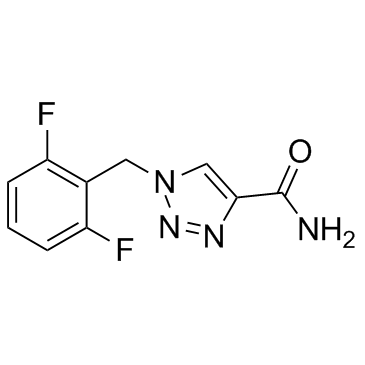
卢非酰胺结构式

|
常用名 | 卢非酰胺 | 英文名 | Rufinamide |
|---|---|---|---|---|
| CAS号 | 106308-44-5 | 分子量 | 238.193 | |
| 密度 | 1.5±0.1 g/cm3 | 沸点 | 473.8±55.0 °C at 760 mmHg | |
| 分子式 | C10H8F2N4O | 熔点 | 232-234?C | |
| MSDS | 中文版 美版 | 闪点 | 240.4±31.5 °C |
|
Dose-dependent pharmacokinetics and brain penetration of rufinamide following intravenous and oral administration to rats.
Eur. J. Pharm. Sci. 68 , 106-13, (2015) Rufinamide is a third-generation antiepileptic drug, approved recently as an orphan drug for the treatment of Lennox-Gastaut syndrome. Although extensive research was conducted, its pharmacokinetics in rats was not described. This work addresses that area by ... |
|
|
Toxicological screening of human plasma by on-line SPE-HPLC-DAD: identification and quantification of basic drugs and metabolites.
Biomed. Chromatogr. 29 , 935-52, (2015) An automated multi-analyte screening method for the identification and quantification of 92 drugs and metabolites based on on-line solid-phase extraction-high-performance liquid chromatography-diode array detection technique was developed and successfully val... |
|
|
Rufinamide for the treatment of Lennox-Gastaut syndrome.
Expert Opin. Pharmacother. 12(5) , 801-6, (2011) Lennox-Gastaut syndrome (LGS) is a severe treatment-resistant childhood-onset epilepsy. This review examines the role of the new drug rufinamide for the treatment of LGS.MEDLINE and Google Scholar searches were undertaken. The pharmaceutical company was conta... |
|
|
Treatment of malignant migrating partial epilepsy of infancy with rufinamide: report of five cases.
Epileptic Disord. 13(1) , 18-21, (2011) The syndrome of malignant migrating partial seizures of infancy (MMPEI) is characterized by early onset of multiple seizures types, highly pharmaco-resistant seizures, and overall poor prognosis. In this study, we investigated retrospectively the efficacy of ... |
|
|
Bioavailability of three rufinamide oral suspensions compared with the marketed 400-mg tablet formulation: results from a randomized-sequence, open-label, four-period, four-sequence crossover study in healthy subjects.
Clin. Ther. 33(1) , 146-57, (2011) Rufinamide is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in patients aged ≥4 years.The primary purpose of this study was to compare the relative bioavailability and other pharmacokinetics of rufinamide administe... |
|
|
Rufinamide from clinical trials to clinical practice in the United States and Europe.
Epileptic Disord. 13 Suppl 1 , S27-43, (2011) Rufinamide is a triazole derivative structurally unrelated to other antiepileptic drugs that is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients aged ≥4 years. Originally granted orphan drug status, ... |
|
|
Rufinamide.
Drugs Today (Barc) 43(7) , 455-60, (2007) Rufinamide is a new antiepileptic drug that is effective in acute animal seizure models and also in the kindling model of epilepsy with a high protective index. Its mechanism of action is largely unknown; studies suggest an effect at voltage-gated sodium chan... |
|
|
[Rufinamide. A review of its pharmacokinetic and pharmacodynamic properties].
Rev. Neurol. 47(7) , 369-73, (2008) To review the most important research published on the pharmacokinetic and pharmacodynamic properties of rufinamide (RFM), together with the outcomes of the clinical trials conducted to date with this new antiepileptic drug.RFM is a triazole derivative with n... |
|
|
Short-term efficacy and tolerability of rufinamide adjunctive therapy in children with refractory generalised epilepsy.
Epileptic Disord. 15(1) , 49-54, (2013) We evaluated the efficacy and tolerability of rufinamide adjunctive therapy in children with refractory generalised epilepsy. The study cohort consisted of 20 patients with Lennox-Gastaut syndrome, 5 with Dravet syndrome, and 28 with unclassified refractory g... |
|
|
[Therapeutic drug monitoring of rufinamide].
Therapie. 67(2) , 161-5, (2012) Rufinamide is a third-generation antiepileptic drug, available since early 2010 in France. It is indicated in combination therapy in the Lennox-Gastaut syndrome from the age of 4. It has orphan drug status. The bioavailability of rufinamide is high, but decre... |