戊二酰亚胺
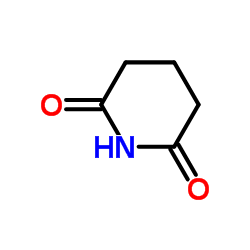
戊二酰亚胺结构式

|
常用名 | 戊二酰亚胺 | 英文名 | Glutarimide |
|---|---|---|---|---|
| CAS号 | 1121-89-7 | 分子量 | 113.115 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 288.0±9.0 °C at 760 mmHg | |
| 分子式 | C5H7NO2 | 熔点 | 155-157 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 152.3±18.9 °C |
|
Comparative characterization of the lactimidomycin and iso-migrastatin biosynthetic machineries revealing unusual features for acyltransferase-less type I polyketide synthases and providing an opportunity to engineer new analogues.
Biochemistry 53(49) , 7854-65, (2014) Lactimidomycin (LTM, 1) and iso-migrastatin (iso-MGS, 2) belong to the glutarimide-containing polyketide family of natural products. We previously cloned and characterized the mgs biosynthetic gene cluster from Streptomyces platensis NRRL 18993. The iso-MGS b... |
|
|
Iso-migrastatin congeners from Streptomyces platensis and generation of a glutarimide polyketide library featuring the dorrigocin, lactimidomycin, migrastatin, and NK30424 scaffolds.
J. Am. Chem. Soc. 127(34) , 11930-1, (2005) Iso-Migrastatin (10) has been shown to be the main natural product of Streptomyces platensis, which undergoes a facile, H2O-mediated rearrangement into dorrigocin A (2), 13-epi-dorrigocin A (11), dorrigocin B (3), and migrastatin (1). Eight new congeners (12-... |
|
|
Enantiomerization mechanism of thalidomide and the role of water and hydroxide ions.
Chemistry 18(45) , 14305-13, (2012) The significance of the molecular chirality of drugs has been widely recognized due to the thalidomide tragedy. Most of the new drugs reaching the market today are single enantiomers, rather than racemic mixtures. However, many optically pure drugs, including... |
|
|
Evaluation of new migrastatin and dorrigocin congeners unveils cell migration inhibitors with dramatically improved potency.
Bioorg. Med. Chem. Lett. 18(22) , 5951-4, (2008) Lactimidomycin (LTM, 1), iso-migrastatin (iso-MGS, 2) and migrastatin (MGS, 3) are macrolide antitumor antibiotics differing in macrolide ring size but all bearing a glutarimide side chain. To further develop these natural products and related analogs as drug... |
|
|
Inhibition of macrophage activation and suppression of graft rejection by DTCM-glutarimide, a novel piperidine derived from the antibiotic 9-methylstreptimidone.
Inflamm. Res. 60(9) , 879-88, (2011) We have previously synthesized a novel piperidine compound, 3-[(dodecylthiocarbonyl)methyl]glutarimide (DTCM-glutarimide), that inhibits LPS-induced NO production, and in the present research we studied further the anti-inflammatory activity of DTCM-glutarimi... |
|
|
Detailed studies on the enantioselective synthesis and HPLC enantioseparation of N-protected 3-hydroxyglutarimides.
Chirality 17(9) , 595-9, (2005) An analytical HPLC method using CHIREX (S)-LEU/(S)-alpha-NEA column was developed for the determination of the enantiomeric excesses of N-protected (S)-3-hydroxyglutarimides. Using this method, detailed studies on the base-promoted ring-expansion reaction of ... |
|
|
Stereoselective aldol reaction of glutarimides using pseudo C(2) symmetry.
Org. Lett. 12(2) , 268-71, (2010) The boron aldol reaction of beta-substituted glutaric imides bearing an oxazolidinone-based auxiliary proceeds with excellent diastereoselectivity; switching the tertiary amine employed between i-Pr(2)EtN or Et(3)N affords enantiomeric lactone product. |
|
|
Theoretical elucidation of the rhodium-catalyzed [4 + 2] annulation reactions.
J. Comput. Chem. 29(5) , 686-93, (2008) The reaction mechanism of the Rh-catalyzed [4 + 2] annulation of 4-alkynals with isocyanates is unraveled using density functional calculations. The reaction mechanisms of the model system and the real substituted system have been investigated and the results... |
|
|
Synthesis and antiviral activities of synthetic glutarimide derivatives.
Chem. Pharm. Bull. 58(11) , 1436-41, (2010) A series of novel glutarimide compounds were synthesized and their antiviral activities were evaluated. The compounds displaying the strongest antiviral activities included 5, 6f, 7e and 9 against coxsackievirus B3 (Cox B3), 10 and 6f against influenza virus ... |
|
|
Solid-phase synthesis of tailed cyclic RGD peptides using glutamic acid: unexpected glutarimide formation.
J. Pept. Sci. 14 , 690-696, (2008) To provide multiple conjugating sites on cyclic peptides for their increasing biomedical applications, a tailed cyclic RGD peptide, c[RGDfE(GGGKK-NH(2))] was designed with c(RGDfE) linked through Glu to a tail consisting of a spacer of three Gly residues and ... |