2-萘甲醚
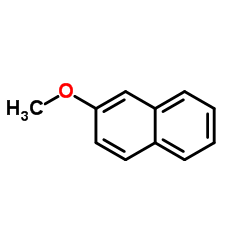
2-萘甲醚结构式

|
常用名 | 2-萘甲醚 | 英文名 | 2-Methoxynaphthalene |
|---|---|---|---|---|
| CAS号 | 93-04-9 | 分子量 | 158.197 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 272.0±0.0 °C at 760 mmHg | |
| 分子式 | C11H10O | 熔点 | 70-73 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 102.3±8.0 °C | |
| 符号 |


GHS07, GHS09 |
信号词 | Warning |
|
Quantitative structure-activity relationship analysis of inhibitors of the nicotine metabolizing CYP2A6 enzyme.
J. Med. Chem. 48 , 440-9, (2005) The purpose of this study was to develop screening and in silico modeling methods to obtain accurate information on the active center of CYP2A6, a nicotine oxidizing enzyme. The inhibitory potencies of 26 naphthalene and 16 non-naphthalene derivatives were de... |
|
|
Exploring QSAR and QAAR for inhibitors of cytochrome P450 2A6 and 2A5 enzymes using GFA and G/PLS techniques
Eur. J. Med. Chem. 44 , 1941-51, (2009) A series of naphthalene and non-naphthalene derivatives ( n = 42) having cytochrome P450 2A6 and 2A5 inhibitory activities, reported by Rahnasto et al., were subjected to QSAR and QAAR studies. The analyses were performed using electronic, spatial, shape and ... |
|
|
Predictive three-dimensional quantitative structure-activity relationship of cytochrome P450 1A2 inhibitors.
J. Med. Chem. 48 , 3808-15, (2005) The purpose of this study was to determine the cytochrome P450 1A2 (CYP1A2) inhibition potencies of structurally diverse compounds to create a comprehensive three-dimensional quantitative structure-activity relationship (3D-QSAR) model of CYP1A2 inhibitors an... |
|
|
Single-step delamination of a MWW borosilicate layered zeolite precursor under mild conditions without surfactant and sonication.
J. Am. Chem. Soc. 136(4) , 1449-61, (2014) Layered borosilicate zeolite precursor ERB-1P (Si/B = 11) is delaminated via isomorphous substitution of Al for B using a simple aqueous Al(NO3)3 treatment. Characterization by PXRD shows loss of long-range order, and TEM demonstrates transformation of rectil... |
|
|
Alkali-metal-mediated manganation(II) of naphthalenes: constructing metalla-anthracene and metalla-phenanthrene structures.
Inorg. Chem. 48(18) , 8863-70, (2009) Alkali-metal-mediated manganation (AMMMn) reactions of the synergic base sodium monoalkyl-bisamidomanganate [(tmeda)Na(tmp)(CH(2)SiMe(3))Mn(tmp)] (1) with naphthalene, 1-methoxynaphthalene, or 2-methoxynaphthalene are reported. These novel direct manganation ... |
|
|
Oxidation of 2-methoxynaphthalene by toluene, naphthalene and biphenyl dioxygenases:structure and absolute stereochemistry of metabolites.
Bioorg. Med. Chem. 2(7) , 727-34, (1994) 2-Methoxynaphthalene was subjected to biooxidation by whole cells of six organisms: Pseudomonas putida F39/D containing toluene dioxygenase, Escherichia coli JM109(pDTG601), containing recombinant toluene dioxygenase from Pp F39/D, Pseudomonas sp. NCIB 9816/1... |
|
|
Dioxygenation of naphthalene by Pseudomonas fluorescens N3 dioxygenase: optimization of the process parameters.
Biotechnol. Bioeng. 93(3) , 511-8, (2006) The bioconversion of naphthalene to the 1,2-dihydro-1,2-dihydroxy derivative was performed in good yield using an Escherichia coli recombinant strain carrying Pseudomonas fluorescens N3 dioxygenase. However, the efficiency of such transformation is affected b... |
|
|
Entrapment and release characteristics of 2-methoxynaphthalene from cylindrical microstructures formed from phospholipids.
J. Microencapsul. 10(2) , 215-22, (1993) Many natural products that exhibit biocidal activity have poor solubility in water. In order to explore the prolonged delivery of these compounds from microtubules we have utilized 2-methoxynaphthalene as a model to elucidate release characteristics of hydrop... |
|
|
Identification of chemicals, possibly originating from misuse of refillable PET bottles, responsible for consumer complaints about off-odours in water and soft drinks.
Food Addit. Contam. 22(7) , 681-92, (2005) Mineral water and soft drinks with a perceptible off-odour were analysed to identify contaminants originating from previous misuse of the refillable polyethylene terephthalate (PET) bottle. Consumers detected the off-odour after opening the bottle and duly re... |
|
|
Synthesis of carbon-11-labeled piperidine ring of N-[omega-(6-methoxynaphthalen-1-yl)alkyl] derivatives as new selective PET sigma1 receptor probes.
Appl. Radiat. Isot. 68(3) , 459-65, (2010) Carbon-11-labeled piperidine ring of N-[omega-(6-methoxynaphthalen-1-yl)alkyl] derivatives were first designed and synthesized as new selective PET sigma(1) receptor probes. The target tracers were prepared by O-[(11)C]methylation of their corresponding pheno... |