1,4-萘二酚
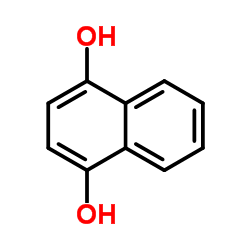
1,4-萘二酚结构式

|
常用名 | 1,4-萘二酚 | 英文名 | BENZOHYDROQUINONE |
|---|---|---|---|---|
| CAS号 | 571-60-8 | 分子量 | 160.169 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 391.0±15.0 °C at 760 mmHg | |
| 分子式 | C10H8O2 | 熔点 | 191-192 °C (dec.) | |
| MSDS | 中文版 美版 | 闪点 | 202.5±15.0 °C | |
| 符号 |


GHS05, GHS07 |
信号词 | Danger |
|
2,3-Disubstituted-1,4-naphthoquinones, 12H-benzo[b]phenothiazine-6,11-diones and related compounds: synthesis and biological evaluation as potential antiproliferative and antifungal agents.
Eur. J. Med. Chem. 44 , 1086-92, (2009) A series of 2-chloro-3-arylsulfanyl-[1,4]naphthoquinones (2), 2,3-bis-arylsulfanyl-[1,4]naphthoquinones (3) and 12H-benzo[b]phenothiazine-6,11-diones and their analogs 6-8 were synthesized and evaluated for their antiproliferative activity against human cervi... |
|
|
A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity
Bioorg. Med. Chem. 20(14) , 4472-81, (2012) Nuclear monoamination of a 1,4-naphthohydroquinone with primary aromatic amines was catalysed by the commercial laccase, Novozym 51003, from Novozymes to afford aminonaphthoquinones. The synthesis was accomplished by reacting a mixture of the primary amine an... |
|
|
Dual effects of superoxide dismutase on the autoxidation of 1,4-naphthohydroquinone.
Free Radic. Biol. Med. 8(1) , 21-4, (1990) The autoxidation of 1,4-naphthohydroquinone, in a phosphate, EDTA buffer at pH 7.4, exhibits an autocatalysis whose lag phase becomes more pronounced in the presence of either the Cu,Zn- or the Mn-containing superoxide dismutases. In contrast, the autoxidatio... |
|
|
Autoxidation of naphthohydroquinones: effects of metals, chelating agents, and superoxide dismutase.
Free Radic. Biol. Med. 22(4) , 689-95, (1997) At neutral pH, 1,4-naphthohydroquinone and 2-methyl-1,4-naphthohydroquinone readily autoxidize to the corresponding quinones. In an unpurified phosphate buffer, the autoxidation of both substances proceeded in a linear fashion after a brief lag phase. Additio... |
|
|
Formation of epoxide and quinone protein adducts in B6C3F1 mice treated with naphthalene, sulfate conjugate of 1,4-dihydroxynaphthalene and 1,4-naphthoquinone.
Arch. Toxicol. 69(6) , 362-7, (1995) Naphthalene (NA) is metabolically activated to the reactive intermediates, naphthalene oxide (NO) and naphthoquinones. To investigate the role of circulating reactive metabolites in NA toxicity, the half-life of NO was examined. The in vitro half-life of NO i... |
|
|
Determination of dihydroxynaphthalenes in human urine by gas chromatography-mass spectrometry.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 826(1-2) , 206-13, (2005) A gas chromatography-mass spectrometry (GC-MS) method was developed for measuring 1,2-dihydroxynaphthalene (1,2-DHN) and 1,4-dihydroxynaphthalene (1,4-DHN) in urine. The method involves enzymatic digestion of urinary conjugates to release the DHNs which were ... |
|
|
N-Heterocyclic carbene-catalyzed monoacylation of 1,4-naphthoquinones with aldehydes.
J. Org. Chem. 74(24) , 9573-5, (2009) The NHC-catalyzed conjugate hydroacylation of 1,4-naphthoquinones allows for the synthesis of monoacylated 1,4-dihydroxynaphthalene derivatives. These targets, difficult to prepare selectively by standard protocols, represent important intermediates in the el... |
|
|
Autoxidation of naphthohydroquinones: effects of pH, naphthoquinones and superoxide dismutase.
Free Radic. Res. 32(3) , 245-53, (2000) The rates of autoxidation of a number of pure naphthohydroquinones have been determined, and the effects of pH, superoxide dismutase (SOD) and of the parent naphthoquinone on the oxidation rates have been investigated. Most compounds were slowly oxidised in a... |
|
|
Synthesis and cytotoxic evaluation of 6-(3-pyrazolylpropyl) derivatives of 1,4-naphthohydroquinone-1,4-diacetate.
Arch. Pharm. (Weinheim) 342(10) , 591-9, (2009) Several new 6-(3-pyrazolylpropyl) derivatives of 1,4-naphthohydroquinone-1,4-diacetate (NHQ-DA) have been prepared by chemical modifications of the Diels-Alder adduct of alpha-myrcene and 1,4-benzoquinone. All these new compounds and precursors have been eval... |
|
|
Selective nonpeptidic inhibitors of herpes simplex virus type 1 and human cytomegalovirus proteases.
Biol. Pharm. Bull. 24(3) , 236-41, (2001) The proteases encoded by herpesviruses including herpes simplex virus type 1 (HSV-1) and human cytomegalovirus (HCMV) are attractive targets for antiviral drug development because of their important roles in viral replication. We randomly screened a chemical ... |