硫辛酰胺
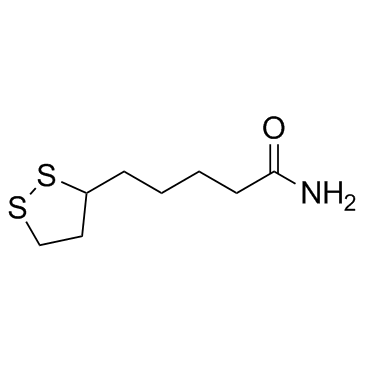
硫辛酰胺结构式

|
常用名 | 硫辛酰胺 | 英文名 | Lipoamide |
|---|---|---|---|---|
| CAS号 | 940-69-2 | 分子量 | 205.34100 | |
| 密度 | 1.169 g/cm3 | 沸点 | 399.2ºC at 760 mmHg | |
| 分子式 | C8H15NOS2 | 熔点 | 127-129ºC(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 195.2ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity.
Cell 159(7) , 1615-25, (2014) Sirtuins (SIRTs) are critical enzymes that govern genome regulation, metabolism, and aging. Despite conserved deacetylase domains, mitochondrial SIRT4 and SIRT5 have little to no deacetylase activity, and a robust catalytic activity for SIRT4 has been elusive... |
|
|
Molecular characterization of the thioredoxin system from Methanosarcina acetivorans.
FEBS J. 281(20) , 4598-611, (2014) The thioredoxin system, composed of thioredoxin reductase (TrxR) and thioredoxin (Trx), is widely distributed in nature, where it serves key roles in electron transfer and in the defense against oxidative stress. Although recent evidence reveals Trx homologue... |
|
|
Catalysis of diaphorase reactions by Mycobacterium tuberculosis lipoamide dehydrogenase occurs at the EH4 level.
Biochemistry 42(7) , 2218-28, (2003) Lipoamide dehydrogenase catalyzes the reversible NAD(+)-dependent oxidation of the dihydrolipoyl cofactors that are covalently attached to the acyltransferase components of the pyruvate dehydrogenase, alpha-ketoglutarate dehydrogenase, and glycine reductase m... |
|
|
Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase.
Biochem. Biophys. Res. Commun. 225(1) , 268-74, (1996) Reduction of the antioxidant lipoic acid has been proposed to be catalyzed in vivo by lipoamide dehydrogenase (LipDH) or glutathione reductase (GR). We have found that thioredoxin reductase (TR) from calf thymus, calf liver, human placenta, and rat liver effi... |
|
|
Ligand-induced conformational changes and a reaction intermediate in branched-chain 2-oxo acid dehydrogenase (E1) from Thermus thermophilus HB8, as revealed by X-ray crystallography.
J. Mol. Biol. 337(4) , 1011-33, (2004) The alpha(2)beta(2) tetrameric E1 component of the branched-chain 2-oxo acid (BCOA) dehydrogenase multienzyme complex is a thiamin diphosphate (ThDP)-dependent enzyme. E1 catalyzes the decarboxylation of a BCOA concomitant with the formation of the alpha-carb... |
|
|
Alpha lipoic acid inhibits human T-cell migration: implications for multiple sclerosis.
J. Neurosci. Res. 78(3) , 362-70, (2004) We have demonstrated previously the ability of the antioxidant alpha lipoic acid (ALA) to suppress and treat a model of multiple sclerosis (MS), relapsing experimental autoimmune encephalomyelitis (EAE). We describe the effects of ALA and its reduced form, di... |
|
|
Roles of His291-alpha and His146-beta' in the reductive acylation reaction catalyzed by human branched-chain alpha-ketoacid dehydrogenase: refined phosphorylation loop structure in the active site.
J. Biol. Chem. 278(44) , 43402-10, (2003) We report here that alterations of either His291-alpha or His146-beta' in the active site of human branched-chain alpha-ketoacid dehydrogenase (E1b) impede both the decarboxylation and the reductive acylation reactions catalyzed by E1b as well as the binding ... |
|
|
Reaction mechanism for mammalian pyruvate dehydrogenase using natural lipoyl domain substrates.
Arch. Biochem. Biophys. 386(2) , 123-35, (2001) The pyruvate dehydrogenase (E1) component of the pyruvate dehydrogenase complex (PDC) catalyzes a two-step reaction. Recombinant production of substrate amounts of the lipoyl domains of the dihydrolipoyl transacetylase (E2) component of the mammalian PDC allo... |
|
|
Alpha-lipoic acid and alpha-lipoamide prevent oxidant-induced lysosomal rupture and apoptosis.
Redox Rep. 6(5) , 327-34, (2001) Alpha-lipoic acid (LA) and its corresponding derivative, alpha-lipoamide (LM), have been described as antioxidants, but the mechanisms of their putative antioxidant effects remain largely uncharacterised. The vicinal thiols present in the reduced forms of the... |
|
|
Amino-terminal residues 1-45 of the Escherichia coli pyruvate dehydrogenase complex E1 subunit interact with the E2 subunit and are required for activity of the complex but not for reductive acetylation of the E2 subunit.
Biochemistry 43(44) , 14037-46, (2004) While N-terminal amino acids 1-55 are not seen in the structure of the Escherichia coli pyruvate dehydrogenase complex E1 subunit (PDHc-E1), mass spectrometric analysis indicated that this amino-terminal region of PDHc-E1 was protected by PDHc-E2. Hence, five... |