3-硝基苯硼酸
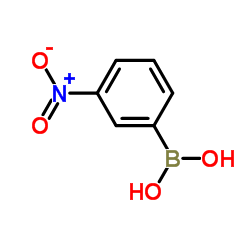
3-硝基苯硼酸结构式

|
常用名 | 3-硝基苯硼酸 | 英文名 | 3-Nitrophenylboronic acid |
|---|---|---|---|---|
| CAS号 | 13331-27-6 | 分子量 | 166.93 | |
| 密度 | 1.4±0.1 g/cm3 | 沸点 | 363.3±44.0 °C at 760 mmHg | |
| 分子式 | C6H6BNO4 | 熔点 | 284-285 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 173.5±28.4 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Boronate affinity-assisted MEKC separation of highly hydrophilic urinary nucleosides using imidazolium-based ionic liquid type surfactant as pseudostationary phase.
Electrophoresis 36(5) , 784-95, (2015) In this work, we extend our investigations regarding the separation of urinary nucleosides by MEKC with the ionic liquid type surfactant 1-tetradecyl-3-methylimidazolium bromide (C14MImBr). We study the impact of adding alkyl- and arylboronic acids (in the pr... |
|
|
A pharmacological MRI assessment of dizocilpine (MK-801) in the 3-nitroproprionic acid-lesioned rat.
Neurosci. Lett. 444(1) , 42-7, (2008) The NMDA-antagonist dizocilpine (MK-801) is known to have dissociative, neurotoxic and neuroprotective properties. Although its neuroprotective properties are well documented, at present only ex vivo autoradiography has demonstrated its activity in lesioned b... |
|
|
Boronic acid catalyzed ene carbocyclization of acetylenic dicarbonyl compounds.
Chem. Commun. (Camb.) 46 , 2191, (2010) The discovery and development of an efficient ene carbocyclization of 1,3-dicarbonyl compounds bearing pendent terminal alkyne substituents under 3-nitrobenzeneboronic acid catalysis is described. The reaction is efficient, easy to perform and general to a wi... |
|
|
Nitrophenylboronic acids as highly chemoselective probes to detect hydrogen peroxide in foods and agricultural products.
J. Agric. Food Chem. 59(21) , 11403-6, (2011) Hydrogen peroxide is commonly used in the food processing industry as a chlorine-free bleaching and sterilizing agent, but excessive amounts of residual hydrogen peroxide have led to cases of food poisoning. Here we describe the development of a novel nonenzy... |
|
|
Direct analysis of polyols using 3-nitrophenylboronic acid in capillary electrophoresis: thermodynamic and electrokinetic principles of molecular recognition.
Anal. Bioanal. Chem 398(3) , 1349-56, (2010) The design of boronic acid sensors for photometric detection of carbohydrates has relied on exploiting differences in the thermodynamic stability of complex formation for molecular recognition. Herein, we introduce a direct method for analysis of sugar alcoho... |
|
|
Kinetic evidence for high reactivity of 3-nitrophenylboronic acid compared to its conjugate boronate ion in reactions with ethylene and propylene glycols.
Inorg. Chem. 47(5) , 1417-9, (2008) The rate constants for a boronate ion were determined for the first time using the reaction systems of 3-nitrophenylboronic acid (3-NO2PhB(OH)2) with ethylene glycol (EG) and propylene glycol (PG) in an alkaline solution: the rate constants (25 degrees C, I =... |
|
|
Parallel synthesis of oxazolines and thiazolines by tandem condensation-cyclodehydration of carboxylic acids with amino alcohols and aminothiols.
J. Comb. Chem. 4(6) , 656-60, (2002) A combinatorial library of oxazolines and thiazolines was synthesized in moderate to excellent yields using a newly developed methodology. Free carboxylic acids were directly condensed with amino alcohols and aminothiols in the presence of 3-nitrophenylboroni... |
|
|
Electrokinetic probes for single-step screening of polyol stereoisomers: the virtues of ternary boronate ester complex formation.
Chem. Commun. (Camb.) (3) , 338-40, (2008) Electrokinetic probes based on the differential migration of ternary boronate ester complexes permit the selective analysis of micromolar levels of UV-transparent polyol stereoisomers in urine samples via dynamic complexation-capillary electrophoresis that is... |
|
|
Aminophenyl- and nitrophenyl-labeled nucleoside triphosphates: synthesis, enzymatic incorporation, and electrochemical detection.
Angew. Chem. Int. Ed. Engl. 47(11) , 2059-62, (2008)
|
|
|
Crystal structures of KPC-2 β-lactamase in complex with 3-nitrophenyl boronic acid and the penam sulfone PSR-3-226.
Antimicrob. Agents Chemother. 56(5) , 2713-8, (2012) Class A carbapenemases are a major threat to the potency of carbapenem antibiotics. A widespread carbapenemase, KPC-2, is not easily inhibited by β-lactamase inhibitors (i.e., clavulanic acid, sulbactam, and tazobactam). To explore different mechanisms of inh... |