酒石酸溴莫尼定
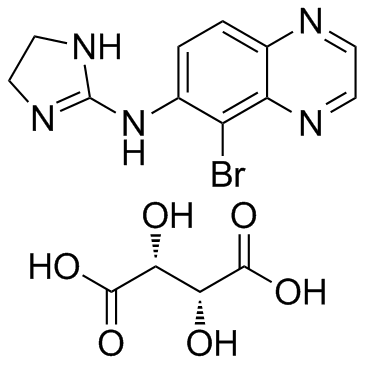
酒石酸溴莫尼定结构式

|
常用名 | 酒石酸溴莫尼定 | 英文名 | Brimonidine L-tartrate |
|---|---|---|---|---|
| CAS号 | 70359-46-5 | 分子量 | 442.221 | |
| 密度 | N/A | 沸点 | 432.6ºC at 760 mmHg | |
| 分子式 | C15H16BrN5O6 | 熔点 | 207-208ºC (dec.) | |
| MSDS | N/A | 闪点 | 215.4ºC | |
| 符号 |

GHS06 |
信号词 | Danger |
|
Dilute brimonidine to improve patient comfort and subconjunctival hemorrhage after LASIK.
J. Refract. Surg. 29(7) , 469-75, (2013) To investigate whether dilute brimonidine (0.025%) reduces patient discomfort, subconjunctival hemorrhage, and injection after LASIK without a significant increase in the rate of flap complications or surgical enhancements.This randomized, double-blind, prosp... |
|
|
Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles.
Invest. Ophthalmol. Vis. Sci. 55(11) , 7387-97, (2014) In this work, we tested the hypothesis that highly targeted delivery of antiglaucoma drugs to the supraciliary space by using a hollow microneedle allows dramatic dose sparing of the drug compared to topical eye drops. The supraciliary space is the most anter... |
|
|
Determinants and characteristics of angle-closure disease in an elderly Chinese population.
Ophthalmic Epidemiol. 22(2) , 109-15, (2015) To determine factors associated with angle-closure disease, particularly in those with structural or functional damage to the eyes, in an elderly Chinese population.A total of 460 individuals aged over 72 years were recruited. The association of angle-closure... |
|
|
Brinzolamide/brimonidine (Simbrinza) for glaucoma.
Med. Lett. Drugs Ther. 55(1421) , 57-8, (2013)
|
|
|
Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2%.
J. Ocul. Pharmacol. Ther. 29(3) , 290-7, (2013) This study compared the intraocular pressure (IOP)-lowering efficacy of fixed-combination brinzolamide 1%/brimonidine 0.2% (BBFC) with that of its component medications, brinzolamide and brimonidine, in patients with open-angle glaucoma or ocular hypertension... |
|
|
Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%.
JAMA Ophthalmol. 131(6) , 724-30, (2013) This study evaluates the contribution of the individual components of an investigational non-β-antagonist fixed combination of brinzolamide, 1%, and brimonidine, 0.2%. This study and its sister study provide the first randomized data showing the intraocular p... |
|
|
Comparison of 24-hour intraocular pressure reduction obtained with brinzolamide/timolol or brimonidine/timolol fixed-combination adjunctive to travoprost therapy.
J. Ocul. Pharmacol. Ther. 29(7) , 652-7, (2013) To determine the adjunctive 24-h efficacy obtained with brinzolamide/timolol, or brimonidine/timolol fixed combinations (FCs) in open-angle glaucoma patients insufficiently controlled on travoprost monotherapy.Prospective, observer-masked, active controlled, ... |
|
|
Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension.
Ophthalmology 121(12) , 2348-55, (2014) To compare the intraocular pressure (IOP)-lowering efficacy and safety of brinzolamide 1% and brimonidine 0.2% fixed combination (BBFC) with that of brinzolamide 1% or brimonidine 0.2% monotherapy, all dosed 2 times per day (BID).Six-month, phase 3, randomize... |
|
|
Neuroprotection for treatment of glaucoma in adults.
Cochrane Database Syst. Rev. 2 , CD006539, (2013) Glaucoma is a heterogeneous group of conditions involving progressive damage to the optic nerve, deterioration of retinal ganglion cells and ultimately visual field loss. It is a leading cause of blindness worldwide. Open angle glaucoma (OAG), the commonest f... |
|
|
Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study.
Am. J. Ophthalmol. 157(5) , 945-52, (2014) To investigate risk factors for disc hemorrhage detection in the Low-Pressure Glaucoma Treatment Study.Cohort of a randomized, double-masked, multicenter clinical trial.Low-Pressure Glaucoma Treatment Study patients with at least 16 months of follow-up were i... |