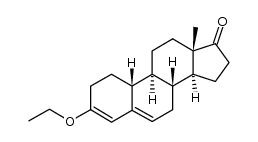
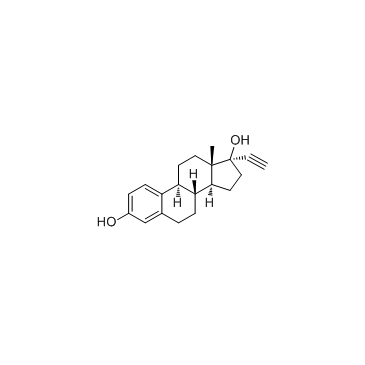
68-22-4
| 中文名 | 炔诺酮 |
|---|---|
| 英文名 | norethisterone |
| 中文别名 |
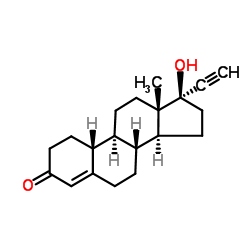
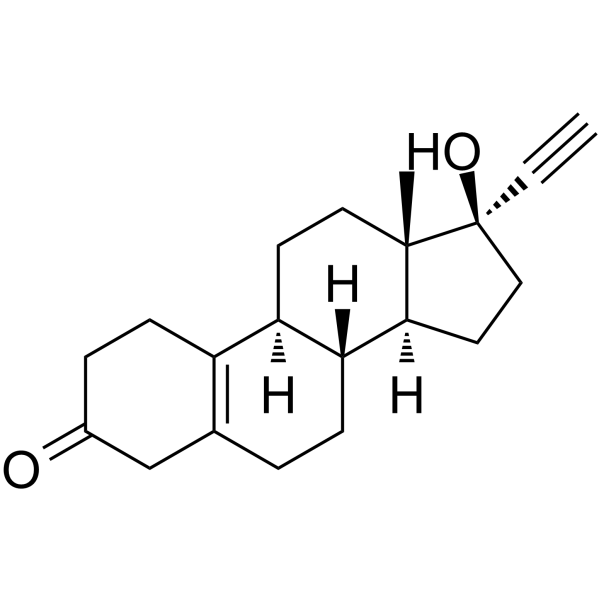
17α-乙炔基-17β-羟基-19-去甲-4-雄甾烯-3-酮
19诺塞睾甾酮 17α-乙炔基-19-去甲睾酮 4-19-去甲基雄烯二醇 去甲基脱氢羟孕酮 17-羟基-19-去甲-17α-孕甾-4-烯-20-炔基-3-酮 氯塞酮 19-去甲-17α-乙炔基-4-雄甾烯-17β-醇-3-酮 |
| 英文别名 |
Mini-Pe
Noriday Ethynylnortestosterone Utovlan sc4640 Anovule MFCD00067596 (17a)-17-Hydroxy-19-norpregn-4-en-20-yn-3-one 19-Nor-17a-ethynyl-17b-hydroxy-4-androsten-3-one 19-nor-17α-Ethynylandrosten-17β-ol-3-one Menzol ENT (8R,9S,10R,13S,14S,17R)-17-ethynyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one 17α-Ethynyl-17β-hydroxyestr-4-en-3-one 19-nor-17α-Ethynyl-17β-hydroxy-4-androsten-3-one Norethindrone Primolut N (17b)-17-ethynyl-17-hydroxyestr-4-en-3-one Ethinylnortestosterone Norfor 17a-Ethynyl-19-nortestosterone (17α)-17-Hydroxy-19-norpregn-4-en-20-yn-3-one 19-Nor-17α-ethynyltestosterone Norgestin 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one 19-Nor-17a-ethynyltestosterone EINECS 200-681-6 (8R,9S,10R,13S,14S,17R)-17-Ethinyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-on 17α-Ethynyl-17-hydroxy-4-estren-3-one 19-Nor-17-ethinyl testosterone Triella 19-Nor-17a-ethynylandrosten-17b-ol-3-one 17α-Ethynyl-19-nortestosterone 17α-Ethynyl-17β-hydroxy-19-norandrost-4-en-3-one Norpregneninolone Norethindrone (USP) norethisterone (8R,9S,10R,13S,14S,17R)-17-éthynyl-17-hydroxy-13-méthyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-3H-cyclopenta[a]phénanthrén-3-one 17β-Hydroxy-19-norpregn-4-en-20-yn-3-one Gestest NET |
| 描述 | Norethindrone 是FDA批准用于治疗子宫内膜异位症,由异常激素水平引起的子宫出血症和继发性闭经的女性孕激素。 |
|---|---|
| 相关类别 | |
| 靶点 |
Progesterone Receptor[1] |
| 体内研究 | 醋酸炔诺酮是治疗症状性子宫内膜异位症的一种经济有效的替代品,副作用相对较轻。接受醋酸炔诺酮治疗的受试者获得痛经和非周期性骨盆疼痛缓解[1]。单用醋酸炔诺酮是治疗子宫内膜异位症所有阶段疼痛和出血的一种耐受性好、有效的选择。醋酸炔诺酮治疗后的出血评分与之前的激素治疗方案无关,所有患者的醋酸炔诺酮治疗后的疼痛评分都有所改善,但之前处方的促性腺激素释放激素激动剂加上补充组除外[2]。醋酸炔诺酮对实验动物的急性毒性较低,与人类的毒性缺乏一致。将醋酸炔诺酮以人类剂量的几倍单独给药于啮齿动物不会导致与治疗相关的死亡率、血液学改变、行为改变或器官病理学改变[3]。给药醋酸炔诺酮可显著且成比例地降低高碳水化合物饮食大鼠密度<1.006的血浆脂蛋白中甘油三酯、胆固醇和磷脂的浓度。醋酸炔诺酮(0.1 mM)也显著抑制了棕榈酸盐和甘油在喂养大鼠肝细胞甘油三酯中的结合[4]。 |
| 动物实验 | 大鼠:雌性Sprague-Dawley大鼠(200-210 g),其中6只作为对照,分别关在上午9:00至晚上9:00照明的动物室中。接受醋酸炔诺酮的7只大鼠中的每只均饲喂35μg/天,持续2周。水和高碳水化合物的大鼠食物可以随意饮用[4]。 |
| 参考文献 |
| 密度 | 1.2±0.1 g/cm3 |
|---|---|
| 沸点 | 447.0±45.0 °C at 760 mmHg |
| 熔点 | 205-206 °C(lit.) |
| 分子式 | C20H26O2 |
| 分子量 | 298.419 |
| 闪点 | 190.5±21.3 °C |
| 精确质量 | 298.193268 |
| PSA | 37.30000 |
| LogP | 3.38 |
| 外观性状 | 灰白色至淡黄色固体 |
| 蒸汽压 | 0.0±2.5 mmHg at 25°C |
| 折射率 | 1.577 |
| 储存条件 | 本品应密封于阴凉干燥处避光保存。 |
| 稳定性 | 具有黄体酮样的孕激素,作用较炔孕酮强4倍,且抑制排卵作用较黄体酮强,还有弱的雄激素和雌激素活性。 |
| 水溶解性 | chloroform: ≥50 mg/mL, clear, colorless |
| 更多 | 1. 性状:白色结晶粉末。无臭微苦 2. 密度(g/mL,25/4℃):不确定 3. 相对蒸汽密度(g/mL,空气=1):不确定 4. 熔点(ºC):205-206 (lit.) 5. 沸点(ºC,常压):不确定 6. 沸点(ºC, 5.2kPa):不确定 7. 折射率:不确定 8. 闪点(ºC):不确定 9. 比旋光度(º):-31.7(氯仿中) 10. 自燃点或引燃温度(ºC):不确定11. 蒸气压(kPa,25ºC):不确定 12. 饱和蒸气压(kPa,60ºC):不确定 13. 燃烧热(KJ/mol):不确定 14. 临界温度(ºC):不确定 15. 临界压力(KPa):不确定 16. 油水(辛醇/水)分配系数的对数值:不确定 17. 爆炸上限(%,V/V):不确定 18. 爆炸下限(%,V/V):不确定 19. 溶解性:不溶于水,微溶于乙醇,略溶于丙酮,溶于氯仿 |
|
Section 1. Chemical Product and Company Identification Norethindrone Common Name/ Trade Name Manufacturer Commercial Name(s) Synonym
Chemical Name Chemical Family Norethindrone Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention if symptoms appear. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionNot available. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various SubstancesNon-flammable in presence of shocks. Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Not available. Special Remarks on Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Norethindrone Section 7. Handling and Storage PrecautionsKeep locked up.. Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not breathe dust. Wear suitable protective clothing. If ingested, seek medical advice immediately and show the container or the label. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Powdered solid.)OdorOdorless. Bitter. (Slight.) Taste Molecular Weight298.4 g/mole White. Color Not applicable. pH (1% soln/water) Boiling PointNot available. Melting Point204°C (399.2°F) Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Water/Oil Dist. Coeff.The product is more soluble in oil; log(oil/water) = 3 Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water, diethyl ether. SolubilityPartially soluble in diethyl ether. Very slightly soluble in acetone. Insoluble in cold water. Section 10. Stability and Reactivity Data StabilityThe product is stable. Not available. Instability Temperature Conditions of InstabilityExcess heat, incompatible materials Incompatibility with various Reactive with oxidizing agents. substances Norethindrone Not available. Corrosivity Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity Will not occur. Polymerization Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsAcute oral toxicity (LD50): 6000 mg/kg [Mouse]. Chronic Effects on HumansMUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female, Reproductive system/toxin/male [SUSPECTED]. May cause damage to the following organs: the reproductive system. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onMay cause cancer. Chronic Effects on HumansMay cause adverse reproductive effects(maternal and paternal effects, effects on male and female fertility) and birth defects. Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: May cause eye irritation. Inhalation: May cause respiratory tract irritation. Ingestion: May cause gastrointestinal tract irritation. May affect endocrine system and skin (dermatitis), and blood. Section 12. Ecological Information EcotoxicityNot available. Not available. BOD5 and COD Possibly hazardous short term degradation products are not likely. However, long term degradation products may Products of Biodegradation arise. Toxicity of the ProductsNot available. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Norethindrone Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms California prop. 65: This product contains the following ingredients for which the State of California has found to Federal and State cause cancer, birth defects or other reproductive harm, which would require a warning under the statute: Regulations Norethindrone California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Norethindrone California prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: Norethindrone Illinois toxic substances disclosure to employee act: Norethindrone Pennsylvania RTK: Norethindrone Minnesota: Norethindrone Massachusetts RTK: Norethindrone California Director's list of Hazardous Substances: Norethindrone CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found Proposition 65to cause cancer which would require a warning under the statute: Norethindrone Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Norethindrone Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)R40- Possible risks of irreversibleS2- Keep out of the reach of children. effects.S36/37- Wear suitable protective clothing and R60- May impair fertility.gloves. S45- In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). S53- Avoid exposure - obtain special instructions before use. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) Norethindrone DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 符号 |

GHS08 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H351 |
| 警示性声明 | P281 |
| 个人防护装备 | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| 危害码 (欧洲) | Xn |
| 风险声明 (欧洲) | R40 |
| 安全声明 (欧洲) | S22-S36/37/39-S45 |
| 危险品运输编码 | 2811.0 |
| WGK德国 | 3 |
| RTECS号 | RC8975000 |
| 海关编码 | 2937290090 |
| 上游产品 9 | |
|---|---|
| 下游产品 7 | |
| 海关编码 | 2937290090 |
|---|