Norethindrone
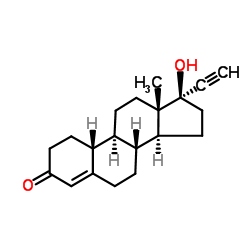
Norethindrone structure
|
Common Name | Norethindrone | ||
|---|---|---|---|---|
| CAS Number | 68-22-4 | Molecular Weight | 298.419 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 447.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C20H26O2 | Melting Point | 205-206 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 190.5±21.3 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of NorethindroneNorethindrone is a female progestin approved by FDA for the treatment of endometriosis, uterine bleeding caused by abnormal hormone levels, and secondary amenorrhea. |
| Name | norethisterone |
|---|---|
| Synonym | More Synonyms |
| Description | Norethindrone is a female progestin approved by FDA for the treatment of endometriosis, uterine bleeding caused by abnormal hormone levels, and secondary amenorrhea. |
|---|---|
| Related Catalog | |
| Target |
Progesterone Receptor[1] |
| In Vivo | Norethindrone acetate could be a cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis. Subjects treated with norethindrone acetate obtain dysmenorrhea and noncyclic pelvic pain relief[1]. Norethindrone acetate alone is a well-tolerated, effective option to manage pain and bleeding for all stages of endometriosis. Post-Norethindrone acetate bleeding scores are improved regardless of prior hormonal regimen, and post-Norethindrone acetate pain scores improved in all patients except for those previously prescribed GnRH-agonist plus add-back[2]. Norethindrone acetate shows low acute toxicity in experimental animals and is consistent with the lack of toxicity seen in humans. Administration of norethindrone acetate alone to rodents at several multiples of the human dose results in no treatment related mortality, hematological changes, behavioral changes, or organ pathology[3]. Norethindrone acetate administration leads to significant and proportional reductions of the concentrations of triglycerides, cholesterol and phospholipids of plasma lipoproteins of density <1.006 of rats fed a high carbohydrate diet. Norethindrone acetate (0.1 mM) also significantly inhibits the incorporation of both palmitate and glycerol into triglycerides of isolated hepatocytes from fed rats[4]. |
| Animal Admin | Rats: Female Sprague-Dawley rats (200-210 g), 6 of which serve as controls, are individually caged in an animal room illuminated from 9:00 a.m. to 9:00 p.m. Each of the 7 rats receiving norethindrone acetate is fed 35 μg/day for 2 weeks. Water and the rat chow which is high in carbohydrate are available ad libitum[4]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 447.0±45.0 °C at 760 mmHg |
| Melting Point | 205-206 °C(lit.) |
| Molecular Formula | C20H26O2 |
| Molecular Weight | 298.419 |
| Flash Point | 190.5±21.3 °C |
| Exact Mass | 298.193268 |
| PSA | 37.30000 |
| LogP | 3.38 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.577 |
| InChIKey | VIKNJXKGJWUCNN-XGXHKTLJSA-N |
| SMILES | C#CC1(O)CCC2C3CCC4=CC(=O)CCC4C3CCC21C |
| Storage condition | -20°C Freezer |
| Water Solubility | chloroform: ≥50 mg/mL, clear, colorless |
|
Section 1. Chemical Product and Company Identification Norethindrone Common Name/ Trade Name Manufacturer Commercial Name(s) Synonym
Chemical Name Chemical Family Norethindrone Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention if symptoms appear. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionNot available. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various SubstancesNon-flammable in presence of shocks. Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Not available. Special Remarks on Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Norethindrone Section 7. Handling and Storage PrecautionsKeep locked up.. Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not breathe dust. Wear suitable protective clothing. If ingested, seek medical advice immediately and show the container or the label. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Powdered solid.)OdorOdorless. Bitter. (Slight.) Taste Molecular Weight298.4 g/mole White. Color Not applicable. pH (1% soln/water) Boiling PointNot available. Melting Point204°C (399.2°F) Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Water/Oil Dist. Coeff.The product is more soluble in oil; log(oil/water) = 3 Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water, diethyl ether. SolubilityPartially soluble in diethyl ether. Very slightly soluble in acetone. Insoluble in cold water. Section 10. Stability and Reactivity Data StabilityThe product is stable. Not available. Instability Temperature Conditions of InstabilityExcess heat, incompatible materials Incompatibility with various Reactive with oxidizing agents. substances Norethindrone Not available. Corrosivity Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity Will not occur. Polymerization Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsAcute oral toxicity (LD50): 6000 mg/kg [Mouse]. Chronic Effects on HumansMUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female, Reproductive system/toxin/male [SUSPECTED]. May cause damage to the following organs: the reproductive system. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onMay cause cancer. Chronic Effects on HumansMay cause adverse reproductive effects(maternal and paternal effects, effects on male and female fertility) and birth defects. Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: May cause eye irritation. Inhalation: May cause respiratory tract irritation. Ingestion: May cause gastrointestinal tract irritation. May affect endocrine system and skin (dermatitis), and blood. Section 12. Ecological Information EcotoxicityNot available. Not available. BOD5 and COD Possibly hazardous short term degradation products are not likely. However, long term degradation products may Products of Biodegradation arise. Toxicity of the ProductsNot available. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Norethindrone Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms California prop. 65: This product contains the following ingredients for which the State of California has found to Federal and State cause cancer, birth defects or other reproductive harm, which would require a warning under the statute: Regulations Norethindrone California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Norethindrone California prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: Norethindrone Illinois toxic substances disclosure to employee act: Norethindrone Pennsylvania RTK: Norethindrone Minnesota: Norethindrone Massachusetts RTK: Norethindrone California Director's list of Hazardous Substances: Norethindrone CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found Proposition 65to cause cancer which would require a warning under the statute: Norethindrone Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Norethindrone Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)R40- Possible risks of irreversibleS2- Keep out of the reach of children. effects.S36/37- Wear suitable protective clothing and R60- May impair fertility.gloves. S45- In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). S53- Avoid exposure - obtain special instructions before use. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) Norethindrone DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | R40 |
| Safety Phrases | S22-S36/37/39-S45 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | RC8975000 |
| HS Code | 2937290090 |
| Precursor 9 | |
|---|---|
| DownStream 7 | |
| HS Code | 2937290090 |
|---|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
|
Name: Primary qHTS assay for inhibitors of alpha-synuclein gene (SNCA) expression
Source: NCGC
External Id: SNCA-p-activity-luciferase
|
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify ago...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_AG_FLUO8_1536_1X%ACT PRUN
|
|
Name: Cytochrome P450 Family 1 Subfamily A Member 2 (CYP1A2) small molecule antagonists: lu...
Source: 824
External Id: CYP273
|
|
Name: Increase the activity of the Burkholderia fixLJ 2-component system
Source: ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
Target: Burkholderia multivorans
External Id: HMS1625
|
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify pos...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_PAM_FLUO8_1536_1X%ACT PRUN
|
|
Name: qHTS Assay for Small Molecule Inhibitors of the Human hERG Channel Activity
Source: NCGC
External Id: HERG01
|
|
Name: qHTS for Inhibitors of TGF-b: Cytotox Counterscreen
Source: NCGC
Target: N/A
External Id: SMAD3201
|
|
Name: uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay
Source: Burnham Center for Chemical Genomics
Target: cystic fibrosis transmembrane conductance regulator [Homo sapiens]
External Id: SBCCG-A764-CF-PAF-Primary-Assay
|
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify ant...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_ANT_FLUO8_1536_1X%INH PRUN
|
| Mini-Pe |
| Noriday |
| Ethynylnortestosterone |
| Utovlan |
| sc4640 |
| Anovule |
| MFCD00067596 |
| (17a)-17-Hydroxy-19-norpregn-4-en-20-yn-3-one |
| 19-Nor-17a-ethynyl-17b-hydroxy-4-androsten-3-one |
| 19-nor-17α-Ethynylandrosten-17β-ol-3-one |
| Menzol |
| ENT |
| (8R,9S,10R,13S,14S,17R)-17-ethynyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one |
| 17α-Ethynyl-17β-hydroxyestr-4-en-3-one |
| 19-nor-17α-Ethynyl-17β-hydroxy-4-androsten-3-one |
| Norethindrone |
| Primolut N |
| (17b)-17-ethynyl-17-hydroxyestr-4-en-3-one |
| Ethinylnortestosterone |
| Norfor |
| 17a-Ethynyl-19-nortestosterone |
| (17α)-17-Hydroxy-19-norpregn-4-en-20-yn-3-one |
| 19-Nor-17α-ethynyltestosterone |
| Norgestin |
| 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one |
| 19-Nor-17a-ethynyltestosterone |
| EINECS 200-681-6 |
| (8R,9S,10R,13S,14S,17R)-17-Ethinyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-on |
| 17α-Ethynyl-17-hydroxy-4-estren-3-one |
| 19-Nor-17-ethinyl testosterone |
| Triella |
| 19-Nor-17a-ethynylandrosten-17b-ol-3-one |
| 17α-Ethynyl-19-nortestosterone |
| 17α-Ethynyl-17β-hydroxy-19-norandrost-4-en-3-one |
| Norpregneninolone |
| Norethindrone (USP) |
| norethisterone |
| (8R,9S,10R,13S,14S,17R)-17-éthynyl-17-hydroxy-13-méthyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-3H-cyclopenta[a]phénanthrén-3-one |
| 17β-Hydroxy-19-norpregn-4-en-20-yn-3-one |
| Gestest |
| NET |
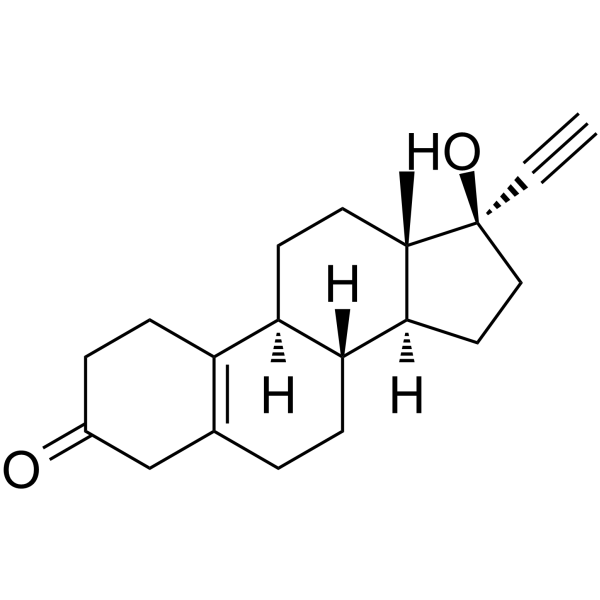 CAS#:68-23-5
CAS#:68-23-5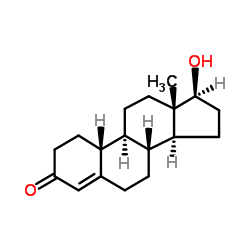 CAS#:434-22-0
CAS#:434-22-0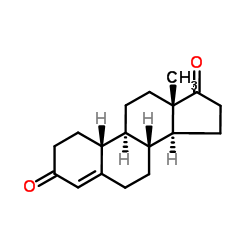 CAS#:734-32-7
CAS#:734-32-7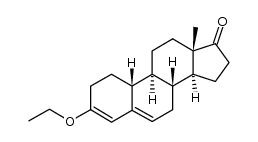 CAS#:2863-88-9
CAS#:2863-88-9 CAS#:74-86-2
CAS#:74-86-2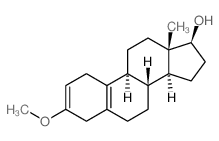 CAS#:1091-93-6
CAS#:1091-93-6 CAS#:75863-04-6
CAS#:75863-04-6 CAS#:1624-62-0
CAS#:1624-62-0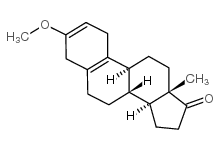 CAS#:17976-32-8
CAS#:17976-32-8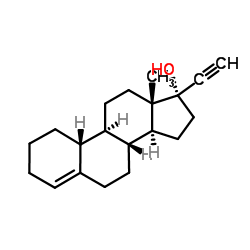 CAS#:52-76-6
CAS#:52-76-6 CAS#:52-78-8
CAS#:52-78-8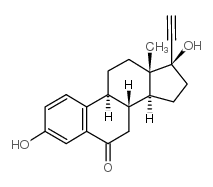 CAS#:38002-18-5
CAS#:38002-18-5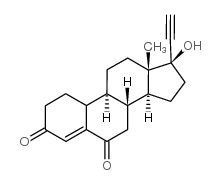 CAS#:67696-78-0
CAS#:67696-78-0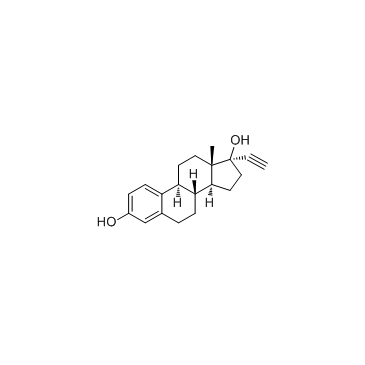 CAS#:57-63-6
CAS#:57-63-6 CAS#:51-98-9
CAS#:51-98-9
