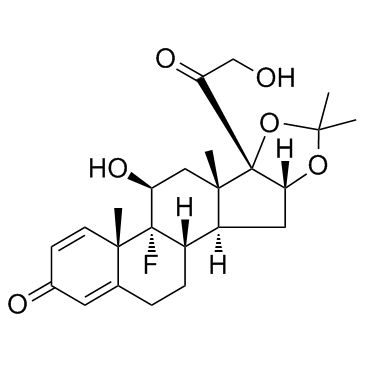
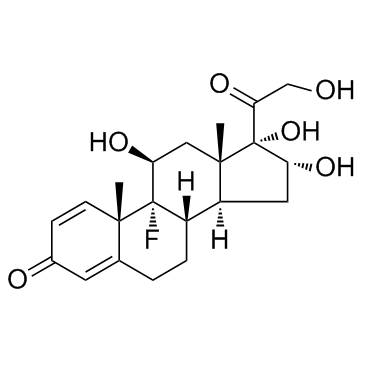
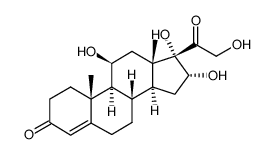
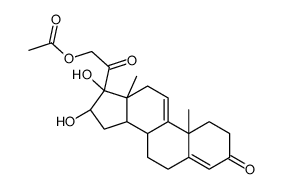
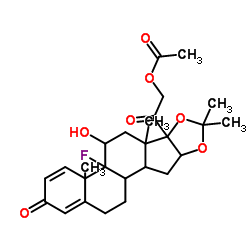
76-25-5
| 中文名 | 曲安奈德 |
|---|---|
| 英文名 | triamcinolone acetonide |
| 中文别名 |
9-氟-11b,21-二羟基-16a,17-[(1-甲基亚乙基)双(氧)]-孕甾-1,4-二烯-3,20-二酮
9-氟-11β,21-二羟基-16α,17-[(1-甲基亚乙基)双(氧)]-孕甾-1,4-二烯-3,20-二酮 醋酸曲安缩松 去炎松-A 曲安缩松 确炎舒松 |
| 英文别名 |
Omcilon-A
(4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-Fluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one (11b,16a)-9-Fluoro-11,21-dihydroxy-16,17-[1-methylethylidenebis(oxy)]pregna-1,4-diene-3,20-dione 9-Fluoro-11,21-dihydroxy-16,17-[1-methylethylidenebis(oxy)]pregna-1,4-diene-3,20-dione 9a-Fluoro-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione Cyclic 16,17-Acetal with Acetone NASACORT VOLON A Kenacort-A Panolog Ointment MFCD00056834 Tramacin 9a-Fluoro-16a,17-isopropylidenedioxyprednisolone Solodelf (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-fluoro-5-hydroxy-6b-(hydroxyacétyl)-4a,6a,8,8-tétraméthyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodécahydro-2H-naphto[2',1':4,5]indéno[1,2-d][1,3]dioxol-2-one 9a-Fluoro-11b,21-dihydroxy-16a,17a-isopropylidenedioxy-1,4-pregnadiene-3,20-dione Adcortyl-A respicort Triam (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-Fluor-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-on Triamcinolone acetonide acetate EINECS 200-948-7 Triamcinolone 16a,17-Acetonide (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-fluoro-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one Rineton Triamcinolone Acetonide Azmacort volonimat 9a-Fluoro-16a-hydroxyprednisolone Acetonide DELPHICORT Kenaquart Kenalog Triamcinolone (acetonide) |
| 描述 | Triamcinolone Acetonide的有效性是prednisone的八倍。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 576.9±50.0 °C at 760 mmHg |
| 熔点 | 274-278ºC (dec.) |
| 分子式 | C24H31FO6 |
| 分子量 | 434.498 |
| 闪点 | 302.7±30.1 °C |
| 精确质量 | 434.210480 |
| PSA | 93.06000 |
| LogP | 2.50 |
| 外观性状 | 白色至灰白色结晶粉末 |
| 蒸汽压 | 0.0±3.6 mmHg at 25°C |
| 折射率 | 1.589 |
| 储存条件 | 本品应密封于阴凉干燥处避光保存。 |
| 分子结构 | 1、 摩尔折射率:109.41 2、 摩尔体积(m3/mol):324.8 3、 等张比容(90.2K):882.9 4、 表面张力(dyne/cm):54.5 5、 极化率(10 -24cm 3):43.37 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:2 3.氢键受体数量:7 4.可旋转化学键数量:2 5.互变异构体数量:9 6.拓扑分子极性表面积93.1 7.重原子数量:31 8.表面电荷:0 9.复杂度:925 10.同位素原子数量:0 11.确定原子立构中心数量:8 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:白色结晶性粉末。 2. 密度(g/mL,25/4℃):不确定 3. 相对蒸汽密度(g/mL,空气=1):不确定 4. 熔点(ºC):274-278°C 5. 沸点(ºC,常压):不确定 6. 沸点(ºC,5.2kPa):不确定 7. 折射率:不确定 8. 闪点(ºC):不确定 9. 比旋光度(º):不确定 10. 自燃点或引燃温度(ºC):不确定 11. 蒸气压(kPa,25ºC):不确定 12. 饱和蒸气压(kPa,60ºC):不确定 13. 燃烧热(KJ/mol):不确定 14. 临界温度(ºC):不确定 15. 临界压力(KPa):不确定 16. 油水(辛醇/水)分配系数的对数值:不确定 17. 爆炸上限(%,V/V):不确定 18. 爆炸下限(%,V/V):不确定 19. 溶解性:不确定 |
|
Section 1. Chemical Product and Company Identification Triamcinolone acetonideCatalog YY1127, TR107 Common Name/ Number(s). Trade Name CAS#76-25-5
Manufacturer RTECSTU3920000 SPECTRUM CHEMICAL MFG. CORP. TSCATSCA 8(b) inventory: Triamcinolone acetonide Commercial Name(s)Not available. CI# Not available. Synonym9-Fluoro-11,21-dihydroxy-16,17-[1-methylethylidenebis(oxy)pregna-1,4-diene-3,20-dione IN CASE OF EMERGENCY Chemical Name Chemical FamilyNot available.CALL (310) 516-8000 C24H31FO6 Chemical Formula SPECTRUM CHEMICAL MFG. CORP. Section 2.Composition and Information on Ingredients Exposure Limits TWA (mg/m3)STEL (mg/m3) CEIL (mg/m3) NameCAS #% by Weight 1) Triamcinolone acetonide76-25-5100 Toxicological DataTriamcinolone acetonide on IngredientsLD50: Not available. LC50: Not available. Section 3. Hazards Identification Potential Acute Health Effects Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Slightly hazardous in case of skin contact (permeator). Potential Chronic HealthCARCINOGENIC EFFECTS: Not available. EffectsMUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: PROVEN The substance is toxic to the reproductive system. Repeated or prolonged exposure to the substance can produce target organs damage. Triamcinolone acetonide Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. Do not use an eye ointment. Seek medical attention. Skin ContactAfter contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin with running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. Cover the irritated skin with an emollient. If irritation persists, seek medical attention. Wash contaminated clothing before reusing. Serious Skin ContactWash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek medical attention. InhalationAllow the victim to rest in a well ventilated area. Seek immediate medical attention. Serious InhalationNot available. IngestionDo not induce vomiting. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Seek immediate medical attention. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. These products are carbon oxides (CO, CO2), halogenated compounds. Products of Combustion Fire Hazards in Presence of Not available. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. of Various SubstancesRisks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks onNot available. Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large Spill Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Triamcinolone acetonide Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not breathe dust. Wear suitable protective clothing In case of insufficient ventilation, wear suitable respiratory equipment If you feel unwell, seek medical attention and show the label when possible. Avoid contact with skin and eyes StorageKeep container dry. Keep in a cool place. Ground all equipment containing material. Keep container tightly closed. Keep in a cool, well-ventilated place. Combustible materials should be stored away from extreme heat and away from strong oxidizing agents. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid.OdorNot available. TasteNot available. 434.49 g/mole Molecular Weight ColorNot available. pH (1% soln/water)Not available. Boiling PointDecomposes. Melting Point293°C (559.4°F) Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Not available. Ionicity (in Water) Dispersion PropertiesNot available. Not available. Solubility Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Not available. Conditions of Instability Incompatibility with various Not available. substances Triamcinolone acetonide CorrosivityNon-corrosive in presence of glass. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationNo. Section 11. Toxicological Information Routes of EntryEye contact. Inhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans DEVELOPMENTAL TOXICITY: PROVEN The substance is toxic to the reproductive system. Other Toxic Effects onHazardous in case of skin contact (irritant), of ingestion, of inhalation. HumansSlightly hazardous in case of skin contact (permeator). Special Remarks onNot available. Toxicity to Animals Special Remarks onAnimal: embryotoxic, foetotoxic, passes through the placental barrier. Menstrual disorders in human. Chronic Effects on Humans Special Remarks on other Not available. Toxic Effects on Humans Section 12. Ecological Information Not available. Ecotoxicity BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the ProductsThe products of degradation are more toxic. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste Disposal Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Special Provisions forNot applicable. Transport Triamcinolone acetonide DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms TSCA 8(b) inventory: Triamcinolone acetonide Federal and State Regulations California Proposition 65 Warnings Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). WHMIS (Canada) CLASS D-2A: Material causing other toxic effects (VERY TOXIC). Other Classifications DSCL (EEC)R36/38- Irritating to eyes and skin. Health Hazard HMIS (U.S.A.)2 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 2 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Wear appropriate respirator when ventilation is inadequate. Triamcinolone acetonide SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 符号 |

GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H360 |
| 警示性声明 | P201-P308 + P313 |
| 个人防护装备 | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| 危害码 (欧洲) | T:Toxic |
| 风险声明 (欧洲) | R61 |
| 安全声明 (欧洲) | S53-S45 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| RTECS号 | TU3920000 |
| 海关编码 | 2937229000 |
| 上游产品 9 | |
|---|---|
| 下游产品 2 | |
| 海关编码 | 2937229000 |
|---|