Temsirolimus (CCI-779)
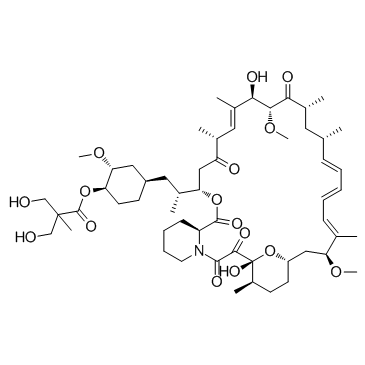
Temsirolimus (CCI-779)结构式

|
常用名 | Temsirolimus (CCI-779) | 英文名 | Temsirolimus (CCI-779) |
|---|---|---|---|---|
| CAS号 | 162635-04-3 | 分子量 | 1030.287 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 1048.4±75.0 °C at 760 mmHg | |
| 分子式 | C56H87NO16 | 熔点 | 99-101ºC | |
| MSDS | 中文版 美版 | 闪点 | 587.8±37.1 °C |
Temsirolimus (CCI-779)用途Temsirolimus 是 mTOR 抑制剂,IC50 值为 1.76 μM。 |
| 中文名 | 西罗莫司脂化物 |
|---|---|
| 英文名 | Temsirolimus |
| 中文别名 | 替西罗莫司 | 西罗莫司 | 坦西莫司 |
| 英文别名 | 更多 |
| 描述 | Temsirolimus 是 mTOR 抑制剂,IC50 值为 1.76 μM。 |
|---|---|
| 相关类别 | |
| 靶点 |
mTOR:1.76 μM (IC50) |
| 体外研究 | Temsirolimus有效抑制mTOR激酶活性,IC50为1.76μM,类似于雷帕霉素,IC50为1.74μM,不存在FKBP12。 Temsirolimus(10 nM至<5μM)通过FKBP12依赖性机制显示适度和选择性的抗增殖活性,但可以在低微摩尔浓度(5-15μM)下完全抑制广泛的肿瘤细胞增殖,涉及FKBP12非依赖性抑制mTOR信号传导。在微摩尔浓度而非纳摩尔浓度(20μM)下的替西罗莫司治疗导致全球蛋白质合成和多核糖体解体的显着下降,伴随着翻译延伸因子eEF2和翻译起始因子eIF2A的磷酸化的快速增加[1]。替西罗莫司抑制核糖体蛋白S6的磷酸化,在PTEN阳性DU145细胞中比在PTEN阴性PC-3细胞中更有效,并且以浓度依赖性方式抑制两种细胞的细胞生长和克隆形成存活[2]。替西罗莫司(100 ng/mL)有效抑制原代人淋巴细胞白血病(ALL)细胞的增殖并诱导细胞凋亡[3]。 |
| 体内研究 | CCI-779(20mg/kg,ip)抑制两种前列腺癌异种移植物的生长,并且以剂量依赖性方式抑制PC-3肿瘤的生长,并且生长抑制大于DU145肿瘤[2]。在含有人ALL的NOD/SCID异种移植模型中,10mg/kg /天的替西罗莫司治疗导致外周血细胞减少和脾肿大[3]。与对照相比,施用西罗莫司脂素(20mg/kg,腹膜内注射5天/周)显着延迟DAOY异种移植物在1周后的生长160%和2周后240%的生长。单次高剂量的替西罗莫司(100mg/kg,ip)治疗在1周内诱导37%的肿瘤体积消退。 Temsirolimus治疗2周也使雷帕霉素抗性U251异种移植物的生长延迟了148%[4]。替西罗莫司对mTOR的抑制改善了四种不同行为任务的表现,并减少了亨廷顿病小鼠模型中的聚集体形成[5]。施用西罗莫司脂诱导显着的剂量依赖性抗肿瘤反应,针对8226,OPM-2和U266异种移植物的皮下生长,ED50为20mg/kg,2mg/kg分别为8226和OPM-2,其与抑制有关。增殖和血管生成,诱导细胞凋亡和减少肿瘤细胞大小[6]。 |
| 激酶实验 | 将Flag标记的野生型人mTOR(Flag-mTOR)DNA构建体瞬时转染到HEK293细胞中。 Flag-mTOR的蛋白质提取和纯化在48小时后进行。在96孔板中进行在不含FKBP12的各种浓度的替西罗莫司存在下纯化的Flag-mTOR的体外激酶测定,并使用His6-S6K1作为底物通过解离增强的镧系元素荧光免疫测定法(DELFIA)进行检测。首先将酶稀释在激酶测定缓冲液(10mM Hepes(pH 7.4),50mM NaCl,50mMβ-甘油磷酸盐,10mM MnCl 2,0.5mM DTT,0.25μM微囊藻毒素LR和100μg/ mL BSA)中。向每个孔中,将12μL稀释的酶与0.5μLTemsirolimus短暂混合。通过添加含有ATP和His6-S6K的12.5μL激酶测定缓冲液来启动激酶反应,得到最终反应体积为25μL,含有800ng / mL FLAG-mTOR,100μMATP和1.25μMHis6-6-S6K。将反应板在室温下温和振荡温育2小时(线性,1-6小时),然后通过加入25μL终止缓冲液(20mM Hepes(pH 7.4),20mM EDTA和20mM EGTA)终止反应板。使用用铕-N1-ITC(Eu)(每个抗体10.4EU)标记的单克隆抗-P(T389)-p70S6K抗体在室温下进行磷酸化(Thr-389)His6-S6K的DELFIA检测。将45μL终止的激酶反应混合物转移至含有55μLPBS的MaxiSorp板。使His6-S6K附着2小时,然后吸出孔并用PBS洗涤一次。添加100μL含有40ng / mL Eu-P(T389)-S6K抗体的DELFIA缓冲液。在温和搅拌下继续抗体结合1小时。然后吸出孔并用含有0.05%Tween 20(PBST)的PBS洗涤四次。向每个孔中加入100μLDELFIA增强溶液,并在PerkinElmer Victor型号读板器中读板。 |
| 细胞实验 | 在集落形成测定中也测定了各种处理后前列腺癌细胞的存活率。指数生长的细胞暴露于不同剂量的米托蒽醌或多西紫杉醇24小时,或暴露于CCI-779 3天。在该处理后,洗涤细胞并用胰蛋白酶消化。将系列稀释液涂布在6孔板中的5mL培养基中。将板在37℃下在含有5%CO 2和90%湿度的气氛中温育10天。然后将板用亚甲蓝染色,并计数含有> 50个细胞的菌落。 |
| 动物实验 | 为了产生异种移植物,将细胞植入基质胶中;将基质胶储存在-20℃,然后在4℃下在冰上解冻3小时,然后使用。将细胞轻轻重悬于1mL PBS中并在冰上温育5分钟。使用预先移液的移液管将细胞转移至含有1mL基质胶的管中,并将细胞浓度调节至3×107 / mL。使用25号针头将细胞(3×10 6在0.1mL中)皮下注射到小鼠的两侧。当异种移植物生长至直径约5mm的大小时,将动物随机分成10只小鼠的组。进行以下实验:携带PC-3肿瘤的小鼠用CCI-779(每天每千克1,5,10和20mg)或载体溶液处理3或5天,每周3周。携带DU145肿瘤的小鼠仅用CCI-779(20mg / kg /天)或载体溶液处理3周。携带PC-3肿瘤的小鼠接受以下治疗:(a)对照,CCI-779的载体溶液; (b)单独化疗,每周腹膜内注射米托蒽醌1.5 mg / kg或多西紫杉醇10 mg / kg,共3剂; (c)单独使用CCI-779,每天ip注射5或10mg / kg,每周注射3次,持续3周; (4)化疗后接CCI-779。 |
| 参考文献 |
| 密度 | 1.2±0.1 g/cm3 |
|---|---|
| 沸点 | 1048.4±75.0 °C at 760 mmHg |
| 熔点 | 99-101ºC |
| 分子式 | C56H87NO16 |
| 分子量 | 1030.287 |
| 闪点 | 587.8±37.1 °C |
| 精确质量 | 1029.602539 |
| PSA | 241.96000 |
| LogP | 2.96 |
| 外观性状 | white to off-white |
| 蒸汽压 | 0.0±0.6 mmHg at 25°C |
| 折射率 | 1.554 |
| 储存条件 | -20°C Freezer |
| 水溶解性 | Soluble in chloroform, methanol. |
| 安全声明 (欧洲) | 24/25 |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
| RTECS号 | VE6257000 |
| 海关编码 | 29349990 |
| Temsirolimus (CCI-779)上游产品 2 | |
|---|---|
| Temsirolimus (CCI-779)下游产品 0 | |
|
Che-1-induced inhibition of mTOR pathway enables stress-induced autophagy.
EMBO J. 34 , 1214-30, (2015) Mammalian target of rapamycin (mTOR) is a key protein kinase that regulates cell growth, metabolism, and autophagy to maintain cellular homeostasis. Its activity is inhibited by adverse conditions, in... |
|
|
Dual fatty acid synthase and HER2 signaling blockade shows marked antitumor activity against breast cancer models resistant to anti-HER2 drugs.
PLoS ONE 10 , e0131241, (2015) Blocking the enzyme Fatty Acid Synthase (FASN) leads to apoptosis of HER2-positive breast carcinoma cells. The hypothesis is that blocking FASN, in combination with anti-HER2 signaling agents, would b... |
|
|
Dual blockade of PI3K/AKT/mTOR (NVP-BEZ235) and Ras/Raf/MEK (AZD6244) pathways synergistically inhibit growth of primary endometrioid endometrial carcinoma cultures, whereas NVP-BEZ235 reduces tumor growth in the corresponding xenograft models.
Gynecol. Oncol. 138 , 165-73, (2015) Endometrial carcinoma (EC) is the most common gynecological cancer in the Western World. Treatment options are limited for advanced and recurrent disease. Therefore, new treatment options are necessar... |
| CCL-779 |
| TeMsiroliMus (~) |
| Torisel |
| Temsirolimus |
| [14C]-Temsirolimus |
| 42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate]rapamycin |
| Propanoic acid, 3-hydroxy-2-(hydroxymethyl)-2-methyl-, (1R,2R,4S)-2-methoxy-4-[(2R)-2-[(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,26R,27R,34aS)-1,4,5,6,9,10,11,12,13,14,21,22,23,24,25,26,27,28,29,31,32,33,34,34a-tetracosahydro-9,27-dihydroxy-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-1,5,11,28,29-pentaoxo-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontin-3-yl]propyl]cyclohexyl ester |
| Rapamycin (42-[3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate] |
| TeMsiroliMus (Torisel) |
| Rapamycin 42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate |
| rapaMycin 42-ester with 3-hydroxy-2-(hydroxyMethyl)-2-Methylpropionic acid |
| (1R,2R,4S)-4-{(2R)-2-[(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-1,5,11,28,29-pentaoxo-1,4,5,6,9,10,11,12,13,14,21,22,23,24,25,26,27,28,29,31,32,33,34,34a-tetracosahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontin-3-yl]propyl}-2-methoxycyclohexyl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate |
| (1R,2R,4S)-4-{(2R)-2-[(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,35R)-1,18-Dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0] hexatriaconta-16,24,26,28-tetraen-12-yl]propyl}-2-methoxycyclohexyl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate |
| NSC 683864 |

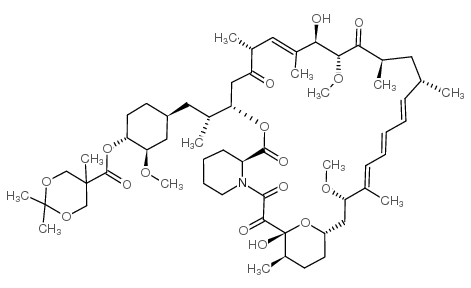 CAS号162635-03-2
CAS号162635-03-2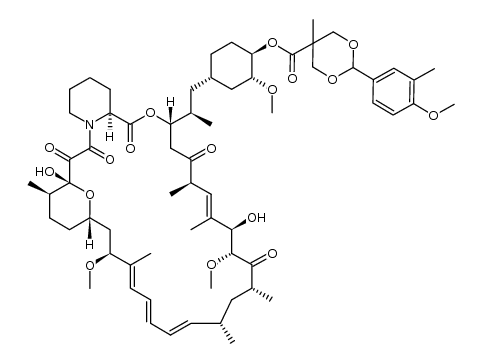 CAS号1316755-20-0
CAS号1316755-20-0
