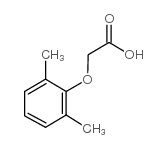
Lopinavir Metabolite M-1
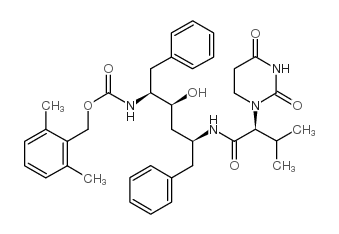
Lopinavir Metabolite M-1结构式

|
常用名 | Lopinavir Metabolite M-1 | 英文名 | Lopinavir Metabolite M-1 |
|---|---|---|---|---|
| CAS号 | 192725-39-6 | 分子量 | 642.78400 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C37H46N4O6 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
Lopinavir Metabolite M-1用途Lopinavir Metabolite M-1 是 Lopinavir 的活性代谢产物,以 0.7 pM 的 Ki 值抑制 HIV 蛋白酶。Lopinavir Metabolite M-1 在体外具有抗病毒活性。 |
| 英文名 | Lopinavir Metabolite M-1 |
|---|---|
| 英文别名 | 更多 |
| 描述 | Lopinavir Metabolite M-1 是 Lopinavir 的活性代谢产物,以 0.7 pM 的 Ki 值抑制 HIV 蛋白酶。Lopinavir Metabolite M-1 在体外具有抗病毒活性。 |
|---|---|
| 相关类别 | |
| 靶点 |
IC50: 0.7 pM (HIV Protease)[1] |
| 体外研究 | 洛比那韦代谢产物M-1对MT-4细胞具有抗病毒活性,EC50为1.413μM[1]。 |
| 参考文献 |
| 分子式 | C37H46N4O6 |
|---|---|
| 分子量 | 642.78400 |
| 精确质量 | 642.34200 |
| PSA | 137.07000 |
| LogP | 5.63440 |
| 折射率 | 1.582 |
|
~81% 
Lopinavir Metab... 192725-39-6 |
| 文献:Sham, Hing L; Betebenner, David A; Herrin, Thomas; Kumar, Gondi; Saldivar, Ayda; Vasavanonda, Sudthida; Molla, Akhter; Kempf, Dale J; Plattner, Jacob J; Norbeck, Daniel W Bioorganic and Medicinal Chemistry Letters, 2001 , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
Lopinavir Metab... 192725-39-6 |
| 文献:Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
Lopinavir Metab... 192725-39-6 |
| 文献:Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
Lopinavir Metab... 192725-39-6 |
| 文献:Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
Lopinavir Metab... 192725-39-6 |
| 文献:Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
Lopinavir Metab... 192725-39-6 |
| 文献:Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
| Lopinavir Metabolite M-1上游产品 6 | |
|---|---|
| Lopinavir Metabolite M-1下游产品 0 | |
| (2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-(2S-(1-tetrahydropyrimid-2,4-dionyl)-3-methyl-butanoyl)amino-1,6-diphenylhexane |
| (aS)-N-[(1S,3S,4S)-4-[[(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2,4-dioxo-1(2H)-Pyrimidineacetami-de |
| (aS)-N-[(1S,3S,4S)-4-[[(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2,4-dioxo-1(2H)-Pyrimidineacetami- d |
| 4-Oxo-ABT-378 |


![N-[(1S,2S,4S)-4-氨基-2-羟基-5-苯基-1-(苯基甲基)戊基]-2-(2,6-二甲基苯氧基)乙酰胺结构式](https://image.chemsrc.com/caspic/013/192725-49-8.png)