Ondansetron HCl
更新时间:2025-08-21 18:40:12
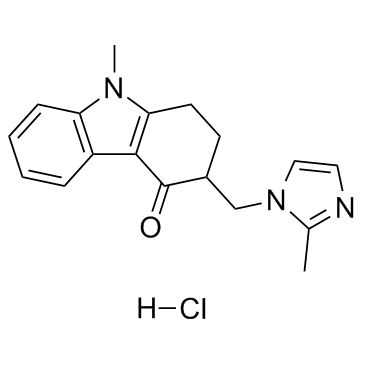
Ondansetron HCl结构式

|
常用名 | Ondansetron HCl | 英文名 | Ondansetron Hydrochloride |
|---|---|---|---|---|
| CAS号 | 99614-01-4 | 分子量 | 329.824 | |
| 密度 | 1.27g/cm3 | 沸点 | 546ºC at 760mmHg | |
| 分子式 | C18H20ClN3O | 熔点 | 178.5-179.5ºC | |
| MSDS | N/A | 闪点 | 284ºC |
Ondansetron HCl用途Ondansetron盐酸盐是5-HT3受体拮抗剂,可作为化疗后止吐剂。 |
| 中文名 | 盐酸枢复宁 |
|---|---|
| 英文名 | ondansetron hydrochloride |
| 中文别名 | 盐酸昂丹司琼 | 帕罗西汀杂质A |
| 英文别名 | 更多 |
| 描述 | Ondansetron盐酸盐是5-HT3受体拮抗剂,可作为化疗后止吐剂。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.27g/cm3 |
|---|---|
| 沸点 | 546ºC at 760mmHg |
| 熔点 | 178.5-179.5ºC |
| 分子式 | C18H20ClN3O |
| 分子量 | 329.824 |
| 闪点 | 284ºC |
| 精确质量 | 329.129486 |
| PSA | 39.82000 |
| LogP | 3.93050 |
| InChIKey | MKBLHFILKIKSQM-UHFFFAOYSA-N |
| SMILES | Cc1nccn1CC1CCc2c(c3ccccc3n2C)C1=O.Cl |
| 外观性状 | 白色粉末 |
| 储存条件 | −20°C |
| 水溶解性 | H2O: >5 mg/mL |
| 危害码 (欧洲) | T: Toxic; |
|---|---|
| 风险声明 (欧洲) | R25 |
| 安全声明 (欧洲) | 45-37/39-26 |
| 危险品运输编码 | UN 2811 6 |
| WGK德国 | 3 |
| RTECS号 | FE6375500 |
| 包装等级 | II |
| 危险类别 | 6.1(a) |
| 海关编码 | 29339900 |
|
~90% 
Ondansetron HCl 99614-01-4 |
| 文献:WO2005/37822 A1, ; Page/Page column 12 ; |
|
~78% 
Ondansetron HCl 99614-01-4 |
| 文献:WO2005/37822 A1, ; Page/Page column 12 ; |
|
~93% 
Ondansetron HCl 99614-01-4 |
| 文献:YUHAN CORPORATION Patent: WO2005/37822 A1, 2005 ; Location in patent: Page/Page column 12 ; |
| Ondansetron HCl上游产品 4 | |
|---|---|
| Ondansetron HCl下游产品 2 | |
| UNII:2999F27MAD |
| (R)-Ondansetron Hydrochloride Dihydrate |
| 4H-CARBAZOL-4-ONE,1,2,3,9-TETRAHYDRO-9-METHYL-3-[(2-METHYL-1H-IMIDAZOL-1-YL)METHYL]-,HYDROCHLORIDE (1:1) |
| 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one |
| LSUREMONOETHANOLAMID |
| Ondansetron HCl |
| 1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one hydrochloride |
| OEA,oleoylethanolamide |
| N-(Hydroxyethyl)oleamide |
| 9-Methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one hydrochloride |
| [3H]-Oleoylethanolamide |
| oleic acid ethanolamide |
| ONDANSETRON HCL DIHYDRATE |
| 9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4h-carbazol-4-onhydrochlorid |
| Ondansetron HCl (Zofran) |
| 4H-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-, monohydrochloride |
| oleic monoethanolamide |
| Ondansetron (hydrochloride) |
| ONDANSETRONHYDROCHLORIDE |
| ONDANSETRON HCL DIHYDRATE IMP. E (EP): 1H-IMIDAZOLE, CRM STANDARD |
| Ondansetron hydrochloride (Zofran) |
| oleic acid-N-monoethanolamide |
| N-(2-Hydroxyethyl)oleamide |
| OEA |
| Oleoylethanolamide |
| [3H]-Ondansetron hydrochloride |
| 4H-Carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-, hydrochloride (1:1) |
| ODANSETRON HYDROCHLORIDE DIHYDRATE |
| Ondansetron Hydrochloride |
| Oleyl monoethanolamide |
| OLEAMIDE MEA |
| 9-méthyl-3-[(2-méthyl-1H-imidazol-1-yl)méthyl]-1,2,3,9-tétrahydro-4H-carbazol-4-one chlorhydrate |
| Oleoylmonoethanolamide |
| 9-Methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one hydrochloride (1:1) |

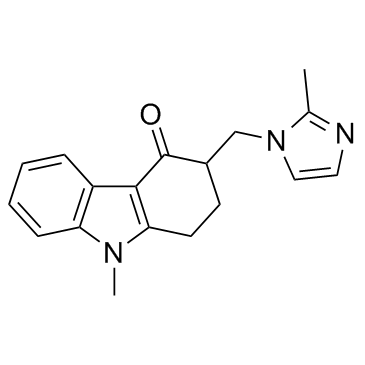


![1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)-methyl]-4H-carbazol-4-one结构式](https://image.chemsrc.com/caspic/094/99614-60-5.png)

