Ginsenoside Rg2
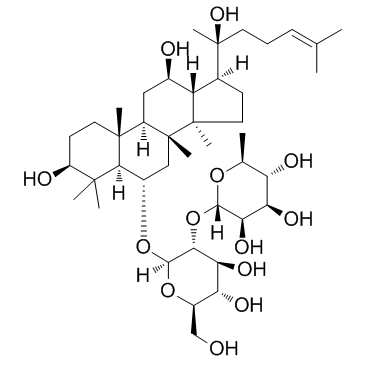
Ginsenoside Rg2 structure
|
Common Name | Ginsenoside Rg2 | ||
|---|---|---|---|---|
| CAS Number | 52286-74-5 | Molecular Weight | 785.013 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 881.0±65.0 °C at 760 mmHg | |
| Molecular Formula | C42H72O13 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 486.6±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Microbial transformation of 20(S)-protopanaxatriol-type saponins by Absidia coerulea.
J. Nat. Prod. 70(7) , 1203-6, (2007) Three 20(S)-protopanaxatriol-type saponins, ginsenoside-Rg1 (1), notoginsenoside-R1 (2), and ginsenoside-Re (3), were transformed by the fungus Absidia coerulea (AS 3.3389). Compound 1 was converted into five metabolites, ginsenoside-Rh4 (4), 3beta,2beta,25-t... |
|
|
Properties of ginseng saponin inhibition of catecholamine secretion in bovine adrenal chromaffin cells.
Eur. J. Pharmacol. 341(2-3) , 139-44, (1998) To investigate the relationship between the inhibitory effects of ginseng saponins (ginsenosides) on acetylcholine-evoked secretion of catecholamines and the structures of ginsenosides, we examined the effects of ginsenoside-Rg3 and -Rh2, which are panaxadiol... |
|
|
Characterization of ginseng saponin ginsenoside-Rg(3) inhibition of catecholamine secretion in bovine adrenal chromaffin cells.
Biochem. Pharmacol. 62(7) , 943-51, (2001) Since ginsenoside-Rg(3), one of the panaxadiol saponins isolated from the ginseng root, significantly inhibited the secretion of catecholamines from bovine adrenal chromaffin cells stimulated by acetylcholine (ACh), the properties of ginsenoside-Rg(3) inhibit... |
|
|
Enzymatic preparation of ginsenosides Rg2, Rh1, and F1 from protopanaxatriol-type ginseng saponin mixture.
Planta Med. 69(3) , 285-6, (2003) During investigations on the hydrolysis of a protopanaxatriol-type saponin mixture by various glycoside hydrolases, it was found that two minor saponins, ginsenosides Rg 2 and Rh 1, were formed in high yields by crude beta-galactosidase from Aspergillus oryza... |
|
|
Enzymatic preparation of ginsenosides Rg2, Rh1, and F1.
Chem. Pharm. Bull. 51(4) , 404-8, (2003) During investigation of the hydrolysis of a protopanaxatriol-type saponin mixture by various glycoside hydrolases, crude preparations of beta-galactosidase from Aspergillus oryzae and lactase from Penicillium sp. were found to produce two minor saponins, gins... |
|
|
Simultaneous enantiomer determination of 20 (R)- and 20 (S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 850(1-2) , 1-6, (2007) 20 (R,S)-Ginsenoside-Rg2, an anti-shock agent, is prescribed as a racemate. To analyze simultaneously the enantiomers of 20 (R)-ginsenoside-Rg2 and 20 (S)-ginsenoside-Rg2 in plasma, a simple and reproducible high-performance liquid chromatographic (HPLC) meth... |
|
|
Enzymatic preparation of genuine prosapogenin, 20(S)-ginsenoside Rh1, from ginsenosides Re and Rg1.
Biosci. Biotechnol. Biochem. 64(12) , 2739-43, (2000) It was found that a lactase preparation from Penicillium sp. nearly quantitatively hydrolyzed ginsenosides Re and Rg1, which are major saponins in roots of Panax ginseng, to a minor saponin, 20(S)-ginsenoside Rh1 [6-O-beta-D-glucopyranosyl-20(S)-protopanaxatr... |
|
|
Determination of ginsenoside Rf and Rg2 from Panax ginseng using enzyme immunoassay.
Chem. Pharm. Bull. 46(7) , 1144-7, (1998) We have developed an enzyme immunoassay (EIA) to quantify trace amounts of ginsenoside Rf (Rf), one of the glycosides of protopanaxatriol from Panax ginseng. A carrier protein of bovine serum albumin (BSA) was coupled to the carbohydrate component of Rf using... |
|
|
Effects of ginsenoside Rg2 on the ultraviolet B-induced DNA damage responses in HaCaT cells.
Naunyn Schmiedebergs Arch. Pharmacol. 382(1) , 89-101, (2010) Our previous study demonstrated the increase in the repair of UVB damage by mRg2, a mixture of ginsenosides containing 60% Rg2 in NIH3T3 cells. In the present study, the effects of purified Rg2 on the repair and apoptosis in ultraviolet B (UVB)-exposed HaCaT ... |
|
|
Effects of ginsenoside Rg2 on the 5-HT3A receptor-mediated ion current in Xenopus oocytes.
Mol. Cells 15(1) , 108-13, (2003) Treatment with ginsenosides, major active ingredients of Panax ginseng, produces a variety of pharmacological or physiological responses with effects on the central and peripheral nervous systems. Recent reports showed that ginsenoside Rg2 inhibits nicotinic ... |