(2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane
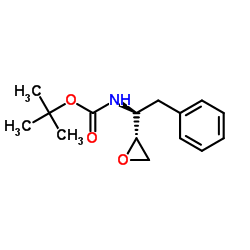
(2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane structure
|
Common Name | (2S,3S)-1,2-Epoxy-3-(Boc-Amino)-4-Phenylbutane | ||
|---|---|---|---|---|
| CAS Number | 98737-29-2 | Molecular Weight | 263.332 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 398.8±25.0 °C at 760 mmHg | |
| Molecular Formula | C15H21NO3 | Melting Point | 125-127ºC | |
| MSDS | Chinese USA | Flash Point | 195.0±23.2 °C | |
| Symbol |
GHS09 |
Signal Word | Warning | |
|
Structure-based design of novel HIV-1 protease inhibitors to combat drug resistance.
J. Med. Chem. 49 , 5252, (2006) Structure-based design and synthesis of novel HIV protease inhibitors are described. The inhibitors are designed specifically to interact with the backbone of HIV protease active site to combat drug resistance. Inhibitor 3 has exhibited exceedingly potent enz... |
|
|
Discovery of HIV-1 protease inhibitors with picomolar affinities incorporating N-aryl-oxazolidinone-5-carboxamides as novel P2 ligands.
J. Med. Chem. 49 , 7342, (2006) Here, we describe the design, synthesis, and biological evaluation of novel HIV-1 protease inhibitors incorporating N-phenyloxazolidinone-5-carboxamides into the (hydroxyethylamino)sulfonamide scaffold as P2 ligands. Series of inhibitors with variations at th... |
|
|
Probing pockets S2-S4' of the gamma-secretase active site with (hydroxyethyl)urea peptidomimetics.
Bioorg. Med. Chem. Lett. 14 , 1935-1938, (2004) (Hydroxyethyl)urea peptidomimetics are potent inhibitors of gamma-secretase that are accessible in a few synthetic steps. Systematic alteration of P2-P4' revealed that the corresponding S2-S4' active site pockets accommodate a variety of substituents, consist... |
|
|
Novel arylsulfonamides possessing sub-picomolar HIV protease activities and potent anti-HIV activity against wild-type and drug-resistant viral strains.
Bioorg. Med. Chem. Lett. 14 , 959-963, (2004) A novel series of P1' chain-extended arylsufonamides was synthesized and evaluated for wild-type HIV protease inhibitory activity and in vitro antiviral activity against wild type virus and two protease inhibitor-resistant mutant viruses. All of the compounds... |