Fenchol
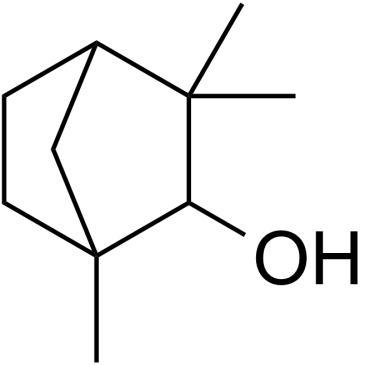
Fenchol structure
|
Common Name | Fenchol | ||
|---|---|---|---|---|
| CAS Number | 1632-73-1 | Molecular Weight | 154.249 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 202.9±8.0 °C at 760 mmHg | |
| Molecular Formula | C10H18O | Melting Point | 35-40ºC | |
| MSDS | Chinese USA | Flash Point | 73.9±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Chocolate smells pink and stripy: Exploring olfactory-visual synesthesia.
Cogn Neurosci 6 , 77-88, (2015) Odors are often difficult to identify, and can be perceived either via the nose or mouth ("flavor"; not usually perceived as a "smell"). These features provide a unique opportunity to contrast conceptual and perceptual accounts of synesthesia. We presented si... |
|
|
[GC-MS analysis and inhibitory activity of the essential oil extracted from the leaves of Lindera communis].
Zhong Yao Cai 22(3) , 128-31, (1999) The essential oil isolated from the dried leaves of Lindera communis was analyzed by means of gas chromatography-mass(GC-MS) technique, the structures of 23 chemical components were identified from it in total, among these, (-)-spathulenol(relative content 22... |
|
|
Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of linalyl pyrophosphate to (-)-endo-fenchol.
J. Biol. Chem. 260(26) , 13901-8, (1985) The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. To test this stereochemical scheme,... |
|
|
Biosynthesis of monoterpenes: conversion of the acyclic precursors geranyl pyrophosphate and neryl pyrophosphate to the rearranged monoterpenes fenchol and fenchone by a soluble enzyme preparation from fennel (Foeniculum vulgare).
Arch. Biochem. Biophys. 200(2) , 524-33, (1980)
|
|
|
Biosynthesis of monoterpenes. Stereochemistry of the enzymatic cyclization of geranyl pyrophosphate to (-)-endo-fenchol.
J. Biol. Chem. 263(30) , 15449-53, (1988) The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. Incubation of (1R)-[2-14C,1-3H]- an... |
|
|
NIR-VCD, vibrational circular dichroism in the near-infrared: experiments, theory and calculations.
Chirality 21 Suppl 1 , E242-52, (2009) The first well documented experiments of Near Infrared Vibrational Circular Dichroism (NIR-VCD) were performed around 1975. We review the thirty year history of NIR-VCD, encompassing both instrumental development and theoretical/computational methods that all... |
|
|
Fragrance material review on fenchyl alcohol.
Food Chem. Toxicol. 46 Suppl 11 , S157-9, (2008) A toxicologic and dermatologic review of fenchyl alcohol when used as a fragrance ingredient is presented. |
|
|
Kinetics and mechanisms of the tropospheric reactions of menthol, borneol, fenchol, camphor, and fenchone with hydroxyl radicals (OH) and chlorine atoms (Cl).
J. Phys. Chem. A 116(16) , 4097-107, (2012) Relative kinetic techniques have been used to measure the rate coefficients for the reactions of oxygenated terpenes (menthol, borneol, fenchol, camphor, and fenchone) and cyclohexanol with hydroxyl radicals (OH) and chlorine atoms (Cl) at 298 ± 2 K and atmos... |
|
|
Roles of human CYP2A6 and rat CYP2B1 in the oxidation of (+)-fenchol by liver microsomes.
Xenobiotica 37(9) , 943-53, (2007) The metabolism of (+)-fenchol was investigated in vitro using liver microsomes of rats and humans and recombinant cytochrome P450 (P450 or CYP) enzymes in insect cells in which human/rat P450 and NADPH-P450 reductase cDNAs had been introduced. The biotransfor... |