1-Adamantyl isocyanate
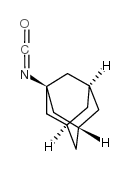
1-Adamantyl isocyanate structure
|
Common Name | 1-Adamantyl isocyanate | ||
|---|---|---|---|---|
| CAS Number | 4411-25-0 | Molecular Weight | 177.24300 | |
| Density | 1.34 g/cm3 | Boiling Point | 247.1ºC at 760 mmHg | |
| Molecular Formula | C11H15NO | Melting Point | 144-146 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 88.5ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Design of bioavailable derivatives of 12-(3-adamantan-1-yl-ureido)dodecanoic acid, a potent inhibitor of the soluble epoxide hydrolase.
Bioorg. Med. Chem. 15(1) , 312-23, (2007) The soluble epoxide hydrolase (sEH) plays an important role in the metabolism of endogenous chemical mediators involved in blood pressure regulation and vascular inflammation. 12-(3-Adamantan-1-yl-ureido)-dodecanoic acid (AUDA, 1) is a very active inhibitor o... |
|
|
Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility.
J. Med. Chem. 47(8) , 2110-22, (2004) The soluble epoxide hydrolase (sEH) is involved in the metabolism of endogenous chemical mediators that play an important role in blood pressure regulation and inflammation. 1,3-Disubstituted ureas are potent inhibitors of sEH that are active both in vitro an... |
|
|
1,3-disubstituted ureas functionalized with ether groups are potent inhibitors of the soluble epoxide hydrolase with improved pharmacokinetic properties.
J. Med. Chem. 50(21) , 5217-26, (2007) Soluble epoxide hydrolase (sEH) is a therapeutic target for treating hypertension and inflammation. 1,3-Disubstituted ureas functionalized with an ether group are potent sEH inhibitors. However, their relatively low metabolic stability leads to poor pharmacok... |
|
|
Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties.
Bioorg. Med. Chem. 21(9) , 2587-99, (2013) Out of the prominent global ailments, tuberculosis (TB) is still one of the leading causes of death worldwide due to infectious disease. Development of new drugs that shorten the current tuberculosis treatment time and have activity against drug resistant str... |
|
|
Nanoparticle vesicles through self assembly of cyclodextrin- and adamantyl-modified silica.
Macromol. Rapid Commun. 31(24) , 2121-6, (2010) Stable nanoparticle vesicles were for the first time prepared from adamantyl- and cyclodextrin (CD)-modified silica nanoparticles forming host-guest interactions in aqueous solution. Adamantyl-functionalized nanoparticles were obtained from thiol-isocyanate r... |