6-Chlorouracil
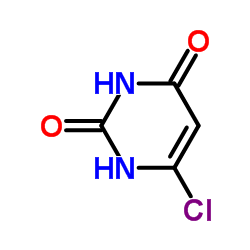
6-Chlorouracil structure
|
Common Name | 6-Chlorouracil | ||
|---|---|---|---|---|
| CAS Number | 4270-27-3 | Molecular Weight | 146.532 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 300°C | |
| Molecular Formula | C4H3ClN2O2 | Melting Point | 290-295°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Electron attachment to chlorouracil: a comparison between 6-ClU and 5-ClU.
J. Chem. Phys. 120 , 704-709, (2004) Low energy electron impact to the isomers 6-chlorouracil (6-ClU) and 5-chlorouracil (5-ClU) yields a variety of negative ion fragments with surprisingly high cross sections. These ions are dominantly formed via sharply structured resonance features at energie... |
|
|
Influence of halogenation on the properties of uracil and its noncovalent interactions with alkali metal ions. Threshold collision-induced dissociation and theoretical studies.
J. Am. Chem. Soc. 126 , 16217-16226, (2004) The influence of halogenation on the properties of uracil and its noncovalent interactions with alkali metal ions is investigated both experimentally and theoretically. Bond dissociation energies of alkali metal ion-halouracil complexes, M+(XU), are determine... |
|
|
Syntheses, pi-stacking interactions and base-pairings of uracil pyridinium salts and uracilyl betaines with nucleobases.
Org. Biomol. Chem. 4 , 3056-3066, (2006) Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaine... |
|
|
Photochemical transformation of 6-chlorouracil and some alkylated analogues.
Biochim. Biophys. Acta 254 , 157-166, (1971)
|
|
|
Tumor uptake of radiolabelled pyrimidine bases and pyrimidine nucleosides in animal models--V. 6-[36Cl]chlorouracil, 6-[82Br]bromouracil and 6-[123I]iodouracil.
Int. J. Nucl. Med. Biol. 11(3-4) , 262-6, (1984)
|
|
|
Divergent synthesis of novel 9-deazaxanthine derivatives via late-stage cross-coupling reactions.
Org. Biomol. Chem. 10(44) , 8860-7, (2012) A small library of 8-substituted 9-deazaxanthines has been prepared by late-stage diversification of an 8-bromo-9-deazaxanthine. By utilizing palladium-catalyzed cross-coupling reactions a single key precursor can be transformed into a variety of 8-substitute... |