5-Indolol
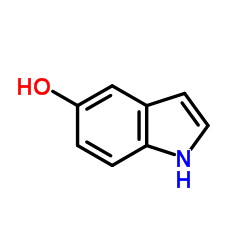
5-Indolol structure
|
Common Name | 5-Indolol | ||
|---|---|---|---|---|
| CAS Number | 1953-54-4 | Molecular Weight | 133.147 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 343.2±15.0 °C at 760 mmHg | |
| Molecular Formula | C8H7NO | Melting Point | 105-110ºC | |
| MSDS | Chinese USA | Flash Point | 161.4±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Validation and application of an UPLC-MS/MS method for the quantification of synthetic cannabinoids in urine samples and analysis of seized materials from the Portuguese market.
Forensic Sci. Int. 243 , 117-25, (2014) An UPLC-MS/MS method using ESI+ionization and MRM was developed and fully validated according to international guidelines for the qualitative and quantitative analysis of nine synthetic cannabinoids and/or their metabolites in urine samples (1mL). Prior to ex... |
|
|
A separation of tyramine on a 2-(4-methoxyphenyl)ethylamine imprinted polymer: an answer from theoretical and experimental studies.
Talanta 129 , 155-64, (2014) A 2-(4-methoxyphenyl)ethylamine imprinted polymer (MIP) was successfully applied for the selective separation of tyramine. A computational analysis was used to predict the affinity of the polymer matrix towards tyramine and a preliminary experimental evaluati... |
|
|
Determination of selected synthetic cannabinoids and their metabolites by micellar electrokinetic chromatography--mass spectrometry employing perfluoroheptanoic acid-based micellar phase.
Talanta 150 , 568-76, (2016) Perfluoroheptanoic acid was employed as a volatile micellar phase in background electrolyte for micellar electrokinetic chromatography-tandem mass spectrometry separation and determination of 15 selected naphthoyl- and phenylacetylindole- synthetic cannabinoi... |
|
|
Stereoselective effect of kynurenine enantiomers on the excretion of serotonin and its metabolite in rat urine.
Chirality 22(2) , 258-61, (2010) A solution of optically pure kynurenine (KYN), i.e., D-KYN or L-KYN, was administered intravenously to male Sprague-Dawley rats (10 mg kg(-1) ml(-1)). The time-course of changes in the concentrations of urinary monoamines and their metabolites such as 5-hydro... |
|
|
[Development of novel fluorescence-derivatization-HPLC methods enabling highly sensitive and selective analysis of biological compounds].
Yakugaku Zasshi 131(8) , 1207-11, (2011) Fluorescence-derivatization-HPLC methods are powerful tools for performing the analysis of bioactive compounds with high sensitivity and selectivity. In this paper, the author reviews the development of the following four types of novel fluorescence-derivatiz... |
|
|
Electronic structure of 5-hydroxyindole: from gas phase to explicit solvation.
J. Phys. Chem. B 113(8) , 2535-41, (2009) We have investigated the absorption and emission spectrum of 5-hydroxyindole in the gas phase and in various solvents. 5-Hydroxyindole is the fluorophore of the non-natural amino acid 5-hydroxytryptophan, which has attracted recent interest as a novel intrins... |
|
|
Positive modulation of alpha7 nAChR responses in rat hippocampal interneurons to full agonists and the alpha7-selective partial agonists, 4OH-GTS-21 and S 24795.
Neuropharmacology 56(4) , 821-30, (2009) One approach for the identification of therapeutic agents for Alzheimer's disease has focused on the research of alpha7 nAChR-selective agonists such as the partial agonists 3-(4-hydroxy,2-methoxybenzylidene)anabaseine (4OH-GTS-21) and, more recently, 2-[2-(4... |
|
|
Modulation of alpha-synuclein aggregation by dopamine analogs.
PLoS ONE 5(2) , e9234, (2010) The action of dopamine on the aggregation of the unstructured alpha-synuclein (alpha-syn) protein may be linked to the pathogenesis of Parkinson's disease. Dopamine and its oxidation derivatives may inhibit alpha-syn aggregation by non-covalent binding. Explo... |
|
|
Synthesis and structure based optimization of 2-(4-phenoxybenzoyl)-5-hydroxyindole as a novel CaMKII inhibitor.
Bioorg. Med. Chem. 20(23) , 6840-7, (2012) Based on 2-(4-phenoxybenzoyl)-5-hydroxyindole (2), a novel structural class of CaMKII inhibitors were synthesized and further optimized. The strong acidity of the hydroxyl group and the lipophilic group at the 4 and 6-positions were found to be necessary for ... |
|
|
Simultaneous determination of 5-hydroxyindoles and catechols from urine using polymer monolith microextraction coupled to high-performance liquid chromatography with fluorescence detection.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 877(20-21) , 1847-55, (2009) To make analytes amenable for fluorescence (FL) detection, polymer monolith microextraction (PMME) coupled to high-performance liquid chromatography with FL detection was developed for the simultaneous determination of catechols and 5-hydroxyindoleamines (5-H... |