Methyl 4-bromobenzoate
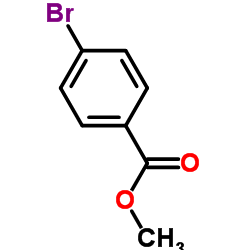
Methyl 4-bromobenzoate structure
|
Common Name | Methyl 4-bromobenzoate | ||
|---|---|---|---|---|
| CAS Number | 619-42-1 | Molecular Weight | 215.044 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 262.2±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H7BrO2 | Melting Point | 77-81 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 112.4±19.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis of classical, three-carbon-bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates.
J. Med. Chem. 47(27) , 6893-6901, (2004) Bridge homologation of the previously reported classical two-carbon-bridged antifolates, a 5-substituted 2,4-diaminofuro[2,3-d]pyrimidine (1) [which is a 6-regioisomer of LY231514 (Alimta)] and a 6-subsituted 2-amino-4-oxopyrrolo[2,3-d]pyrimidine, afforded th... |
|
|
Methyl 4-bromobenzoate. Bolte M and Wissler J.
Acta Crystallogr. Sect. E Struct. Rep. Online 62(3) , o1192-93, (2006)
|
|
|
Zeeman effect on the nuclear quadrupole resonance of 81Br in single crystals of methyl 4-bromobenzoate, 4, 4'-dibromodiphenyl-ether and 4, 4'-dibromodiphenylsulphide at 77 K. Ambrosetti R, et al.
Mol. Phys. 28(2) , 551-8, (1974)
|
|
|
Synthesis and iodination of methyl 4-tri-n-butylstannylbenzoate, p-(methoxycarbonyl) phenylmercuric chloride and p-(methoxycarbonyl) phenylboronic acid. Hylarides MD, et al.
J. Organomet. Chem. 367(3) , 259-65, (1989)
|
|
|
Phosphine-free palladium-catalyzed direct arylation of imidazo [1,2-a] pyridines with aryl bromides at low catalyst loading. Fu HY, et al.
J. Org. Chem. 77(9) , 4473-4478, (2012)
|
|
|
Synthesis of Lithium 2-Pyridyltriolborate and its Cross-Coupling Reaction with Aryl Halides. Yamamoto Y, et al.
Organic Synth. 88 , 79-86, (2011)
|
|
|
Palladium-acetate catalyst for regioselective direct arylation at C2 of 3-furanyl or 3-thiophenyl acrylates with inhibition of Heck type reaction. Chen L, et al.
Tetrahedron 69(22) , 4381-4388, (2013)
|