L(-)-Carvone
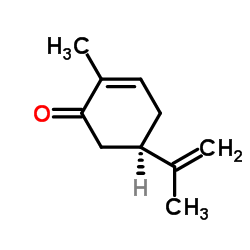
L(-)-Carvone structure
|
Common Name | L(-)-Carvone | ||
|---|---|---|---|---|
| CAS Number | 6485-40-1 | Molecular Weight | 150.218 | |
| Density | 0.958 | Boiling Point | 227-230 ºC | |
| Molecular Formula | C10H14O | Melting Point | 25.2ºC | |
| MSDS | Chinese USA | Flash Point | 88 ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Spasmolytic Activity of Carvone and Limonene Enantiomers.
Nat. Prod. Commun. 10 , 1893-6, (2016) Aromatic plants produce volatile substances with high therapeutic potential. In view of the need for new respiratory and cardiovascular system pharmacological agents, the present study reports on the spasmolytic activity of two enantiomers of carvone and limo... |
|
|
Radical-cation salts of BEDT-TTF with lithium tris(oxalato)metallate(III).
Dalton Trans. 44(13) , 6219-23, (2015) The first radical-cation salts in the extensive family (BEDT-TTF)x[(A)M(C2O4)3]·Guest containing lithium as the counter cation have been synthesized and characterised. |
|
|
Evaluation of the antioxidant and antiproliferative potential of bioflavors
Food Chem. Toxicol. 49(7) , 1610-5, (2011) Highlights ► Antioxidant potential and antiproliferative effect of five monoterpenes was investigated. ► α-Terpineol presented good antioxidant activity in ORAC test. ► Limonene and α-terpineol presented equivalent antiproliferative profile in vitro. ► α-Terp... |
|
|
Changes in Volatile Compounds during Aging of Sweet Fennel Fruits-Comparison of Hydrodistillation and Static Headspace Sampling Methods.
Nat. Prod. Commun. 11 , 423-9, (2016) Two extraction methods for subsequent gas chromatographic (GC) determination of volatiles from freshly harvested and aged fennel fruit samples (Foeniculum vulgare Mill.,ssp. vulgare var. dulce) have been compared. Hydrodistillation followed by GC-FID and GC-M... |
|
|
Zen and the art of molecules.
Nature Chemistry 4(3) , 142-4, (2012)
|
|
|
A convergent stereocontrolled total synthesis of (-)-terpestacin.
Org. Biomol. Chem. 10(28) , 5452-5, (2012) A stereocontrolled total synthesis of (-)-terpestacin has been achieved starting from (R)-(-)-carvone as a chiral pool and (E,E)-farnesol via a highly convergent approach. Thus, (R)-(-)-carvone was transformed into the cyclopentanone segment through a series ... |
|
|
In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens.
Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 29(3) , 415-22, (2012) The aim of this study was to examine the effect of five naturally occurring compounds from essential oils on 10 different species of mycotoxigenic fungi involved in several plant diseases. The antifungal activities of terpinen-4-ol, eugenol, carvone, 1,8-cine... |
|
|
Anxiolytic effects of repeated treatment with an essential oil from Lippia alba and (R)-(-)-carvone in the elevated T-maze.
Braz. J. Med. Biol. Res. 45(3) , 238-43, (2012) Lippia alba (Mill.) N.E. Brown (Verbenaceae) is widely used in different regions of Central and South America as a tranquilizer. The plant's anxiolytic properties, however, merit investigation. The present study evaluated the effects of repeated daily (14 day... |
|
|
Non-natural elemane as the "stepping stone" for the synthesis of germacrane and guaiane sesquiterpenes.
Org. Lett. 15(1) , 152-5, (2013) The synthesis of hydroxyelemane 5 from (R)-carvone and its utilization as a common synthetic scaffold to produce structurally diverse germacrane and guaiane sesquiterpenes are described. A highly enantio- and stereoselective biomimetic tandem oxy-Cope/ene rea... |
|
|
Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species.
Parasitol. Res. 110(5) , 2023-32, (2012) Mosquitoes are blood-feeding insects and serve as the most important vectors for spreading human diseases such as malaria, yellow fever, dengue fever, and filariasis. The continued use of synthetic insecticides has resulted in resistance in mosquitoes. Synthe... |