Tricyclohexylphosphine
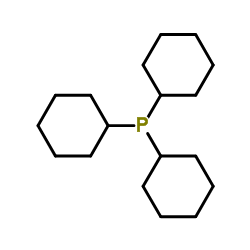
Tricyclohexylphosphine structure
|
Common Name | Tricyclohexylphosphine | ||
|---|---|---|---|---|
| CAS Number | 2622-14-2 | Molecular Weight | 280.428 | |
| Density | 0.909 g/mL at 25 °C | Boiling Point | 383.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C18H33P | Melting Point | 81-83 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 195.6±25.0 °C | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Danger | |
|
Synthesis and Catalytic Performance of Hierarchically Porous MIL-100(Fe)@polyHIPE Hybrid Membranes.
Macromol. Rapid Commun. 36 , 1605-11, (2015) Metal-organic frameworks (MOFs) nanoparticles in combination with a nonionic surfactant (Pluronic L-121) are used to stabilize dicyclopentadiene (DCPD)-in-water high internal phase emulsions (HIPEs). The resulting HIPEs containing the MIL-100(Fe) nanoparticle... |
|
|
Synthesis of tetra-substituted imidazoles and 2-imidazolines by Ni(0)-catalyzed dehydrogenation of benzylic-type imines.
Dalton Trans. 43(42) , 15997-6005, (2014) Ni(0)-catalyzed dehydrogenation of benzylic-type imines was performed to yield asymmetrical tetra-substituted imidazoles and 2-imidazolines. This was achieved with a single operational step while maintaining good selectivity and atom economy. The catalytic sy... |
|
|
Formation of nickeladihydropyran by oxidative addition of cyclopropyl ketone. Key intermediate in nickel-catalyzed cycloaddition.
J. Am. Chem. Soc. 128(6) , 5350-5351, (2006) Cyclopropyl phenyl ketone underwent oxidative addition to Ni(PCy3) generated from Ni(cod)2 and PCy3 to give a nickeladihydropyran, which is a key intermediate for the Ni(0)-catalyzed homo- or heterocycloaddition to give cyclopentane compounds having two carbo... |
|
|
Synlett , 3001, (2006)
|
|
|
Synlett , 3167, (2006)
|