7-hydroxy Warfarin
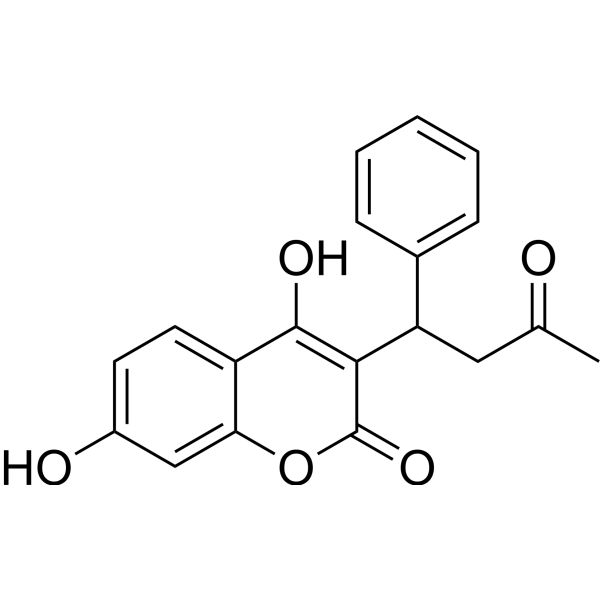
7-hydroxy Warfarin structure
|
Common Name | 7-hydroxy Warfarin | ||
|---|---|---|---|---|
| CAS Number | 17834-03-6 | Molecular Weight | 324.33 | |
| Density | 1.384g/cm3 | Boiling Point | 540.2ºC at 760mmHg | |
| Molecular Formula | C19H16O5 | Melting Point | 220ºC | |
| MSDS | Chinese USA | Flash Point | 197.7ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Novel Marmoset Cytochrome P450 2C19 in Livers Efficiently Metabolizes Human P450 2C9 and 2C19 Substrates, S-Warfarin, Tolbutamide, Flurbiprofen, and Omeprazole.
Drug Metab. Dispos. 43 , 1408-16, (2015) The common marmoset (Callithrix jacchus), a small New World monkey, has the potential for use in human drug development due to its evolutionary closeness to humans. Four novel cDNAs, encoding cytochrome P450 (P450) 2C18, 2C19, 2C58, and 2C76, were cloned from... |
|
|
Investigation of two-dimensional high performance liquid chromatography approaches for reversed phase resolution of warfarin and hydroxywarfarin isomers.
J. Chromatogr. A. 1363 , 200-6, (2014) Several offline and online 2D HPLC methods were investigated for the reversed phase resolution of a complex mixture of closely related warfarin and hydroxywarfarin isomers. By combining reversed phase achiral/chiral HPLC separation with UV-triggered fraction ... |
|
|
Hydroxywarfarin metabolites potently inhibit CYP2C9 metabolism of S-warfarin.
Chem. Res. Toxicol. 23(5) , 939-45, (2010) Coumadin (R/S-warfarin) anticoagulant therapy poses a risk to over 50 million Americans, in part due to interpersonal variation in drug metabolism. Consequently, it is important to understand how metabolic capacity is influenced among patients. Cytochrome P45... |
|
|
Analysis of R- and S-hydroxywarfarin glucuronidation catalyzed by human liver microsomes and recombinant UDP-glucuronosyltransferases.
J. Pharmacol. Exp. Ther. 340(1) , 46-55, (2012) Coumadin (R-, S-warfarin) is a challenging drug to accurately dose, both initially and for maintenance, because of its narrow therapeutic range and wide interpatient variability and is typically administered as a racemic (Rac) mixture, which complicates the b... |
|
|
Development of a method for the analysis of warfarin and metabolites in plasma and urine.
Am. Clin. Lab. 14(7) , 20-1, (1995)
|
|
|
Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9.
Pharmacogenetics 4(1) , 39-42, (1994)
|
|
|
Identification of hydroxywarfarin binding site in human UDP glucuronosyltransferase 1a10: phenylalanine90 is crucial for the glucuronidation of 6- and 7-hydroxywarfarin but not 8-hydroxywarfarin.
Drug Metab. Dispos. 36(11) , 2211-8, (2008) Recent studies show that the extrahepatic human UDP-glucuronosyltransferase (UGT)1A10 is capable of phase II glucuronidation of several major cytochrome P450 metabolites of warfarin (i.e., 6-, 7-, and 8-hydroxywarfarin). This study expands on this finding by ... |
|
|
Determination of warfarin enantiomers and hydroxylated metabolites in human blood plasma by liquid chromatography with achiral and chiral separation.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 818(2) , 191-8, (2005) An assay comprising two simple, selective and isocratic HPLC methods with UV detection was developed and validated for measuring warfarin enantiomers and all five warfarin monohydroxylated metabolites in patient blood plasma. Following liquid/liquid extractio... |
|
|
Substrate probe for the mechanism of aromatic hydroxylation catalyzed by cytochrome P450.
Drug Metab. Dispos. 24(9) , 1038-45, (1996) The effect of branch pathways on the observed intramolecular isotope effect and deuterium retention associated with 6- and 7-hydroxylation of selectively monodeuterated (R)- and (S)-warfarin with cytochrome P450 (CYP) 2C9 and CYP1A2 were studied. cDNA-express... |
|
|
In vitro inhibition of CYP2C9-mediated warfarin 7-hydroxylation by iguratimod: possible mechanism of iguratimod-warfarin interaction.
Biol. Pharm. Bull. 38(3) , 441-7, (2015) Iguratimod is a novel disease-modifying antirheumatic drug. A blue letter (safety advisory) for drug interaction between iguratimod and warfarin was issued by the Ministry of Health, Labour and Welfare of Japan in May 2013. Iguratimod may affect warfarin meta... |