3-Ethynylpyridine
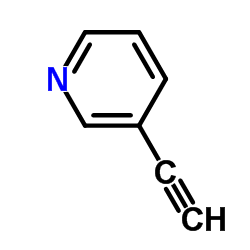
3-Ethynylpyridine structure
|
Common Name | 3-Ethynylpyridine | ||
|---|---|---|---|---|
| CAS Number | 2510-23-8 | Molecular Weight | 103.12 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 170.5±13.0 °C at 760 mmHg | |
| Molecular Formula | C7H5N | Melting Point | 39-40 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 57.5±12.4 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
|
Sequential one-pot ruthenium-catalyzed azide-alkyne cycloaddition from primary alkyl halides and sodium azide.
J. Org. Chem. 76(7) , 2355-9, (2011) An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the... |
|
|
Homochiral supramolecular M2L4 cages by high-fidelity self-sorting of chiral ligands.
Chemistry 19(33) , 10890-4, (2013) A 1,1'-binaphthyl-based bis(pyridine) ligand (1) was prepared in racemic and enantiomerically pure form to study the formation of [Pd2(1)4] complexes upon coordination to palladium(II) ions with regard to the degree of chiral self-sorting. The self-assembly p... |
|
|
Direct use of dioxygen as an oxygen source: catalytic oxidative synthesis of amides.
Chem. Commun. (Camb.) 48(2) , 305-7, (2012) The first transition-metal-catalyzed direct oxidative synthesis of amides by using dioxygen as an oxygen source has been developed under mild conditions, in which DBU was used as the key additive. The present methodology, which utilizes dioxygen as an oxidant... |
|
|
A novel one-pot method for the preparation of pyrazoles by 1,3-dipolar cycloadditions of diazo compounds generated in situ.
J. Org. Chem. 68(13) , 5381-3, (2003) A convenient one-pot procedure for the preparation of pyrazoles by 1,3-dipolar cycloaddition of diazo compounds generated in situ has been developed. Diazo compounds derived from aldehydes were reacted with terminal alkynes to furnish regioselectively 3,5-dis... |