2-Chloro-4-nitropyridine
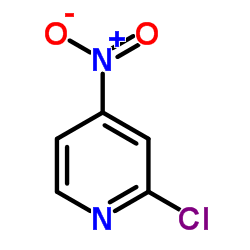
2-Chloro-4-nitropyridine structure
|
Common Name | 2-Chloro-4-nitropyridine | ||
|---|---|---|---|---|
| CAS Number | 23056-36-2 | Molecular Weight | 158.54 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 258.4±20.0 °C at 760 mmHg | |
| Molecular Formula | C5H3ClN2O2 | Melting Point | 52-56 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 110.1±21.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Studies on anticoccidial agents. 13. Synthesis and anticoccidial activity of nitropyridine-2- and -3-sulfonamides and derivatives.
J. Med. Chem. 23(12) , 1376-80, (1980) Eight nitropyridinesulfonamides and pyridinesulfonamide N-oxides as their bioisosteres were prepared and evaluated for anticoccidial activity. Of these compounds, 2-, 4- and 5-nitropyridine-3-sulfonamides and pyridine-2- and -3-sulfonamide N-oxides were found... |
|
|
Tetrabutylammonium salt induced denitration of nitropyridines: synthesis of fluoro-, hydroxy-, and methoxypyridines.
Org. Lett. 7 , 577, (2005) An efficient method for the synthesis of fluoropyridines via the fluorodenitration reaction is reported. The reaction is mediated by tetrabutylammonium fluoride (TBAF) under mild conditions without undue regard to the presence of water. The fluorodenitration ... |
|
|
Diels–Alder cycloadditions of stabilised 2, 3-pyridynes. Connon SJ and Hegarty AF.
Tetrahedron Lett. 42(4) , 735-737, (2001)
|
|
|
Stabilised 2, 3-Pyridyne Reactive Intermediates of Exceptional Dienophilicity. Connon SJ and Hegarty AF.
European J. Org. Chem. 16 , 3477-3483., (2004)
|