trans-2-Methyl-2-butenal
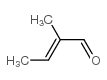
trans-2-Methyl-2-butenal structure
|
Common Name | trans-2-Methyl-2-butenal | ||
|---|---|---|---|---|
| CAS Number | 497-03-0 | Molecular Weight | 84.11640 | |
| Density | 0.871 g/mL at 25 °C(lit.) | Boiling Point | 116-119 °C752 mm Hg(lit.) | |
| Molecular Formula | C5H8O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 65 °F | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
|
Mosquito odorant receptor for DEET and methyl jasmonate.
Proc. Natl. Acad. Sci. U. S. A. 111(46) , 16592-7, (2014) Insect repellents are important prophylactic tools for travelers and populations living in endemic areas of malaria, dengue, encephalitis, and other vector-borne diseases. DEET (N,N-diethyl-3-methylbenzamide) is a 6-decade-old synthetic repellent, which is st... |
|
|
Synthesis of nitidulid beetle pheromones: alkyl-branched tetraene hydrocarbons. Bartelt RJ, et al.
J. Agric. Food Chem. 38(12) , 2192-96, (1990)
|
|
|
Synthesis of 1, 2-and 1, 4-disubstituted tricarbonyl (pentadienyl) iron (+ 1) cations and reactions with heteroatom nucleophiles. Donaldson WA, et al
Organometallics 12(4) , 1174-79, (1993)
|
|
|
Titanium(II)-mediated cyclizations of (silyloxy)enynes: a total synthesis of (-)-7-demethylpiericidin A1.
J. Am. Chem. Soc. 128(2) , 408-9, (2006) A concise total synthesis of 7-demethylpiericidin A1 has been completed. The synthesis features a titanium(II)-mediated cyclization of a (silyloxy)enyne as the key step and proceeds in nine steps from tiglic aldehyde. |
|
|
Characterization and synthesis of volatile compounds from the defensive secretions of some "daddy longlets" (Arachnida: Opiliones: Leiobunum spp.).
Proc. Natl. Acad. Sci. U. S. A. 74(2) , 419-22, (1977) Analyses of the chief volatile constituents of the defensive secretions of three oplionids were carried out. Leiobunum nigripalpi produces three closely related C7 compounds: E-4-methyl-4-hexen-3-one(I), 4-methylhexan-3-one(II), and 4-methylhexan-3-ol(III), a... |