4,4-Dimethoxy-2-butanone
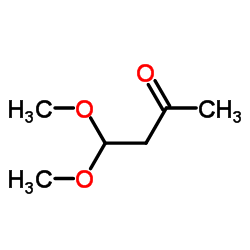
4,4-Dimethoxy-2-butanone structure
|
Common Name | 4,4-Dimethoxy-2-butanone | ||
|---|---|---|---|---|
| CAS Number | 5436-21-5 | Molecular Weight | 132.158 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 172.1±20.0 °C at 760 mmHg | |
| Molecular Formula | C6H12O3 | Melting Point | -82 °C | |
| MSDS | Chinese USA | Flash Point | 49.4±0.0 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
|
Carbonylation as a key reaction in anaerobic acetone activation by Desulfococcus biacutus.
Appl. Environ. Microbiol. 79(20) , 6228-35, (2013) Acetone is activated by aerobic and nitrate-reducing bacteria via an ATP-dependent carboxylation reaction to form acetoacetate as the first reaction product. In the activation of acetone by sulfate-reducing bacteria, acetoacetate has not been found to be an i... |
|
|
Yamadazyma Farinosa IFO 10896-mediated reduction of 4, 4-dimethoxy-2-butanone as the key-step for the preparation of 1, 3-diols with unsymmetrical substituents. Yamazaki T, et al.
Synth. Commun. 30(16) , 3061-72, (2000)
|
|
|
β-keto acetals. I. Synthesis of pyrazoles and pyrimidines and the steric inhibition of resonance in 5-alkyl-1-p-nitrophenylpyrazoles. Burness DM.
J. Org. Chem. 21(1) , 97-101., (1956)
|
|
|
Facile template synthesis of nickel (II) complexes of dibenzotetraaza [14] annulenes. Cutler AR, et al.
Inorg. Chem. 24(14) , 2276-81, (1985)
|
|
|
2-Arylpyrazolo [1,5-a] pyrimidin-3-yl acetamides. New potent and selective peripheral benzodiazepine receptor ligands. Selleri S, et al.
BioTechnol.: Indian J. 9(10) , 2661-2671, (2001)
|
Journals:
More...