n,n'-di-t-butylethylenediamine
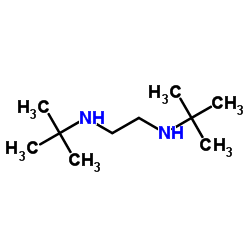
n,n'-di-t-butylethylenediamine structure
|
Common Name | n,n'-di-t-butylethylenediamine | ||
|---|---|---|---|---|
| CAS Number | 4062-60-6 | Molecular Weight | 172.311 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 208.7±8.0 °C at 760 mmHg | |
| Molecular Formula | C10H24N2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 76.7±11.6 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Polymorphism in Lithium Amides: A Structural and Theoretical Study. Synthesis, Mechanism, and NMR Studies of the Lithiation of N,N'-Di-tert-butylethylenediamine.
Inorg. Chem. 35(13) , 4047-4059, (1996) The lithiation of N,N'-di-tert-butylethylenediamine by MeLi in benzene has been shown by (1)H NMR spectroscopy to proceed via the partially lithiated species [cis-{Li[&mgr;-N(t-Bu)CH(2)CH(2)N(H)t-Bu]}(2)], 2, and [{Li[N(t-Bu)CH(2)CH(2)N(H)t-Bu]}(2)Li{N(t-Bu)C... |
|
|
Aluminium amides derived from metallation of N,N'-di-tert-butylethylenediamine. Gardiner MG, et al.
J. Chem. Soc., Dalton Trans. 22 , 4163-69, (1996)
|
|
|
New chemistry from the reaction of N, N'-disubstituted ethylenediamines with glyoxal: synthesis of 2-imidazolidinecarboxaldehydes and 1, 4, 6, 9-tetraalkyl-1, 4, 6, 9-tetraaza-5, 10-dioxaperhydroanthracenes. Willer RL, et al.
J. Org. Chem. 50(13) , 2368-72, (1985)
|
|
|
Silylene and Germylene Intermediates in the Reactions of Silole and Germole Dianions with N,N'-Di-tert-butylethylenediimine. Toulokhonova IS, et al.
Eur. J. Inorg. Chem. 14 , 2344-49, (2008)
|
Journals:
More...