Loteprednol etabonate
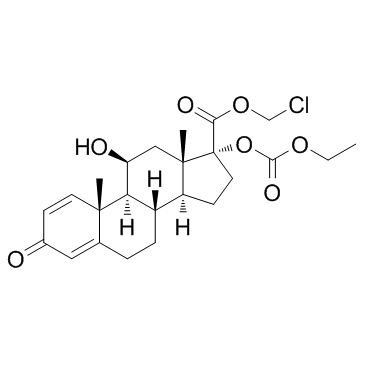
Loteprednol etabonate structure
|
Common Name | Loteprednol etabonate | ||
|---|---|---|---|---|
| CAS Number | 82034-46-6 | Molecular Weight | 466.952 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 600.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C24H31ClO7 | Melting Point | 220.5-223.5ºC | |
| MSDS | Chinese USA | Flash Point | 316.7±31.5 °C | |
|
Topical cyclosporine a 1% for the treatment of chronic ocular surface inflammation.
Eye Contact Lens 40(5) , 283-8, (2014) To evaluate the use of topical cyclosporine A (CsA) 1% emulsion in the treatment of chronic ocular surface inflammation (OSI).We conducted a retrospective chart review of patients with various forms of OSI treated with topical CsA 1% from 2001 to 2012.Twenty-... |
|
|
Small-incision lenticule extraction for myopia: results of a 12-month prospective study.
Optom. Vis. Sci. 92(1) , 123-31, (2015) To analyze the safety, efficacy, stability, and predictability of small-incision lenticule extraction to correct myopia.Patients were evaluated preoperatively and then at 1 day, at 2 weeks, and at 1, 3, 6, and 12 months postoperatively. Safety, efficacy, stab... |
|
|
Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery.
J. Cataract Refract. Surg. 39(2) , 168-73, (2013) To evaluate the efficacy of loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the control of postoperative inflammation in patients having routine cataract surgery.Private practice, Stillwater, Minnesota, and Cincinnati Eye Institute, Cincinnati... |
|
|
Feasibility of localized immunosuppression: 2. PLA microspheres for the sustained local delivery of a soft immunosuppressant.
Pharmazie 65(6) , 429-35, (2010) While biohybrid therapy shows promise, their further development into an "artificial pancreatic" system in diabetics also requires the management of the related immuneresponse triggered by such cellular therapies. Ideally this should be on a local level withi... |
|
|
Pharmacokinetics and delta1-cortienic acid excretion after intravenous administration of prednisolone and loteprednol etabonate in rats.
Pharmazie 65(6) , 412-6, (2010) Detailed pharmacokinetic (PK) studies in rats were performed (i)to compare the PK of prednisolone (PRN) and loteprednol etabonate (LE, a soft corticosteroid) as well as their common inactive metabolite delta1-cortienic acid (delta1-CA), (ii) to investigate th... |
|
|
The effects of delta1-cortienic acid on skin blanching, pharmacokinetics and stability of loteprednol etabonate.
Pharmazie 67(5) , 406-10, (2012) The effect of delta1-cortienic acid (delta1-CA) on human skin blanching activity of the soft corticosteroid, loteprednol etabonate (LE), has been studied. Ten volunteers had applied to their forearms a dose of LE ranging from 0.1 to 1 mM, or LE from 0.1 to 1 ... |
|
|
Exacerbation of zoster interstitial keratitis after zoster vaccination in an adult.
Arch. Ophthal. 128(8) , 1079-80, (2010)
|
|
|
Loteprednol and tobramycin in combination: a review of their impact on current treatment regimens.
Expert Opin. Pharmacother. 11(5) , 843-52, (2010) The treatment of ocular inflammation continues to be a challenge. Topical corticosteroids are effective in reducing ocular inflammation but are limited by adverse events including elevation of intraocular pressure, development of cataracts, glaucoma and inhib... |
|
|
Treatment of seasonal allergic conjunctivitis with ophthalmic corticosteroids: in search of the perfect ocular corticosteroids in the treatment of allergic conjunctivitis.
Curr. Opin. Allergy Clin. Immunol. 10(5) , 469-77, (2010) Corticosteroids are an effective short-term treatment option for seasonal allergic conjunctivitis (SAC). Their use has been limited due to their side effects and has led to the development of modified 'soft', 'smart' ophthalmic corticosteroid formulations tha... |
|
|
Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease.
J. Ocul. Pharmacol. Ther. 27(1) , 23-7, (2011) This retrospective, clinical comparative analysis describes differences in clinical signs and symptoms and medication tolerability between those patients who receive topical corticosteroids prior to initiation of topical cyclosporine 0.5% emulsion (tCSA) ther... |