Dichlormid
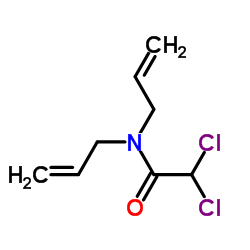
Dichlormid structure
|
Common Name | Dichlormid | ||
|---|---|---|---|---|
| CAS Number | 37764-25-3 | Molecular Weight | 208.085 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 253.8±40.0 °C at 760 mmHg | |
| Molecular Formula | C8H11Cl2NO | Melting Point | 5.0-6.5°C | |
| MSDS | USA | Flash Point | 107.3±27.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Fatty acid elongation is important in the activity of thiocarbamate herbicides and in safening by dichlormid.
J. Exp. Bot. 54(385) , 1289-94, (2003) The thiocarbamates, such as pebulate (S-propyl butyl (ethyl) thiocarbamate) are a well-established class of herbicides. They inhibit fatty acid elongation, which is necessary for the biosynthesis of constituents of surface waxes and suberin and this has been ... |
|
|
The inhibition of fatty acid elongation by pebulate can be effectively counteracted by the safener dichlormid.
Biochem. Soc. Trans. 28(6) , 650-1, (2000) The thiocarbamate herbicide pebulate inhibits fatty acid elongation, which is necessary for surface lipid biosynthesis. As both barley and wild oats are susceptible to pebulate, the safener dichlormid was used to study the reversal of its herbicidal effect. F... |
|
|
Induction of glutathione S-transferases in Arabidopsis by herbicide safeners.
Plant Physiol. 130(3) , 1497-505, (2002) Herbicide safeners increase herbicide tolerance in cereals but not in dicotyledenous crops. The reason(s) for this difference in safening is unknown. However, safener-induced protection in cereals is associated with increased expression of herbicide detoxifyi... |
|
|
Cloning and characterization of maize herbicide safener-induced cDNAs encoding subunits of glutathione S-transferase isoforms I, II and IV.
Plant Mol. Biol. 26(6) , 1855-66, (1994) Several GSTs have been characterised in maize. GST I is a homodimer of 29 kDa subunits, GST II a hetrodimer of 27 kDa and 29 kDa subunits and GST IV a homodimer of 27 kDa subunits. We report the isolation and characterization of a herbicide-safener inducible ... |
|
|
Characterization of the safener-induced glutathione S-transferase isoform II from maize.
Planta 196(2) , 295-302, (1995) The safener-induced maize (Zea mays L.) glutathione S-transferase, GST II (EC 2.5.1.18) and another predominant isoform, GST I, were purified from extracts of maize roots treated with the safeners R-25788 (N,N-diallyl-2-dichloroacetamide) or R-29148 (3-dichlo... |
|
|
Photodegradation of the herbicide EPTC and the safener dichlormid, alone and in combination.
Chemosphere 46(8) , 1183-9, (2002) Photodegradation of the herbicide EPTC (S-ethyl-N, N-dipropylthiocarbamate), and the safener dichlormid (2,2-dichloro-N, N-diallylacetamide) has been examined in methanol and in water solutions. Irradiation of EPTC and dichlormid with UV light at 254 nm cause... |
|
|
Comparative three-dimensional quantitative structure-activity relationship study of safeners and herbicides.
J. Agric. Food Chem. 48(3) , 926-31, (2000) The competitive antagonist hypothesis for safeners and herbicides was investigated by studying the 3D similarity between 28 safener and 20 herbicide molecules in their putative biologically active, low-energy conformations using comparative molecular field an... |
|
|
Effect of safeners on damage of human erythrocytes treated with chloroacetamide herbicides.
Environ. Toxicol. Pharmacol. 36(2) , 368-77, (2013) Chloroacetamides are used as pre-emergent substances for growth control of annual grasses and weeds. Since they can be harmful for crop plants, protective compounds (safeners) are used along with herbicides. So far, their effects on human blood cells have not... |