2-Allylcyclohexanone
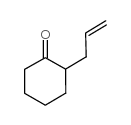
2-Allylcyclohexanone structure
|
Common Name | 2-Allylcyclohexanone | ||
|---|---|---|---|---|
| CAS Number | 94-66-6 | Molecular Weight | 138.20700 | |
| Density | 0.927 g/mL at 25 °C(lit.) | Boiling Point | 94 °C23 mm Hg(lit.) | |
| Molecular Formula | C9H14O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Mn(III)-Based Oxidative Free-Radical Cyclizations of Unsaturated Ketones.
J. Org. Chem. 61(22) , 7832-7847, (1996) Mn(III)-based oxidative free-radical cyclization of unsaturated ketones is a versatile synthetic procedure with broad applicability. For example, oxidation of cyclopentanone 1a with 2 equiv of Mn(OAc)(3).2H(2)O and 1 equiv of Cu(OAc)(2).H(2)O in AcOH at 80 de... |
|
|
A simplified procedure for epoxidation by benzonitrile-hydrogen peroxide. Selective oxidation of 2-allylcyclohexanone. Payne GB.
Tetrahedron 18(6) , 763-65, (1962)
|
|
|
Stereochemical Studies. IX. Asymmetric Synthesis of 2-Alkylcyclo-hexanones with Enamine Alkylation. Hiroi K, et al.
Chem. Pharm. Bull. 20 , 246-57, (1972)
|
|
|
A Modified Thermodynamically Controlled Deracemization of 2-Allylcyclohexanone and Its Application to Asymmetric Synthesis of (R)-(-)-Epilachnene. Kaku H, et al.
Chem. Lett. 33(5) , 516-17, (2004)
|
|
|
Biphase and triphase catalysis. Arsonated polystyrenes as catalysts in the Baeyer-Villiger oxidation of ketones by aqueous hydrogen peroxide. Jacobson SE, et al.
J. Am. Chem. Soc. 101(23) , 6938-46, (1979)
|
Journals:
More...