3-Amino-1,2-propanediol
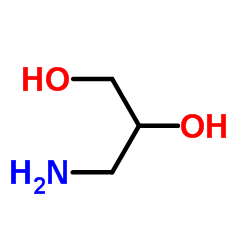
3-Amino-1,2-propanediol structure
|
Common Name | 3-Amino-1,2-propanediol | ||
|---|---|---|---|---|
| CAS Number | 616-30-8 | Molecular Weight | 91.109 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 266.7±20.0 °C at 760 mmHg | |
| Molecular Formula | C3H9NO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 115.1±21.8 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Synthesis and properties of DNA-PNA chimeric oligomers.
Nucleic Acids Res. 24(17) , 3357-63, (1996) Adenine, thymine and cytosine PNA monomers have been prepared using 3-amino-1,2-propanediol as a starting material. The benzoyl group was used to protect the exocyclic amines of the heterocyclic bases of A and C PNA monomers and the backbone primary amine was... |
|
|
Competitive inhibition of lipolytic enzymes. II. Preparation of 'monoacylamino' phospholipids.
Biochim. Biophys. Acta 1043(1) , 67-74, (1990) This paper describes the synthesis of a number of phosphatidylcholines and phosphatidylglycols, in which one fatty acyl ester group is replaced by an acylamino function. The phospholipids, both of the alpha- and beta-type, are prepared in racemic and enantiom... |
|
|
Three new derivatives of 3-amino-1,2-propanediol; their spectral properties and biological evaluation.
Acta Pol. Pharm. 58(4) , 249-56, (2001) Three new derivatives of 3-amino-1,2-propanediol have been synthesized. Full assignments of signals in their 1H- and 13C-NMR spectra are given. The influence of these compounds on the cardiovascular system in the anaesthetized rat was examined. In contrast to... |
|
|
A specific receptor site for glycerol, a new sweet tastant for Drosophila: structure-taste relationship of glycerol in the labellar sugar receptor cell.
Chem. Senses 29(8) , 703-11, (2004) Glycerol, a linear triol, is a sweet tastant for mammals but it has not previously been recognized to stimulate the sense of taste in insects. Here we show by electrophysiological experimentation that it effectively stimulates the labellar sugar receptor cell... |
|
|
A new nanoprobe based on FRET between functional quantum dots and gold nanoparticles for fluoride anion and its applications for biological imaging
Biosens. Bioelectron. 36(1) , 168-73, (2012) A new nanoprobe was designed for the fluorescence imaging of fluoride anion (F−) in living cells with high sensitivity and selectivity. The design is based on the fluorescence resonance energy transfer (FRET) between CdTe quantum dots (CdTe QDs) and gold nano... |
|
|
Conversion of a hemoglobin alpha chain aspartate(47) ester to N-(2,3-dihydroxypropyl)asparagine as a method for identification of the principal binding site for benzo[a]pyrene anti-diol epoxide.
Chem. Res. Toxicol. 4(3) , 359-63, (1991) Human hemoglobin was alkylated with (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE) and then treated with aqueous (+/-)-3-amino-1,2-propanediol to convert alkylated carboxyl side chains to N-(2,3-dihydroxypropyl)... |