2-Acetlimidazole
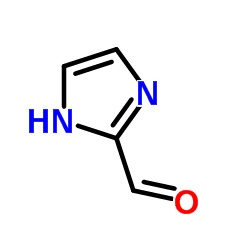
2-Acetlimidazole structure
|
Common Name | 2-Acetlimidazole | ||
|---|---|---|---|---|
| CAS Number | 10111-08-7 | Molecular Weight | 96.09 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 296.5±23.0 °C at 760 mmHg | |
| Molecular Formula | C4H4N2O | Melting Point | 209 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 137.5±29.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Investigation of a Degradant in a Biologics Formulation Buffer Containing L-Histidine.
Pharm. Res. 32 , 2625-35, (2015) An unknown UV 280 nm absorbing peak was observed by SEC for protein stability samples formulated in L-histidine during a stress stability study. Understanding the source would enhance the confidence in the SEC results. We identified the unknown peak, studied ... |
|
|
Mixed ligand copper(II) complexes of 1,10-phenanthroline with tridentate phenolate/pyridyl/(benz)imidazolyl Schiff base ligands: covalent vs non-covalent DNA binding, DNA cleavage and cytotoxicity.
J. Inorg. Biochem. 140 , 255-68, (2014) A series of copper(II) complexes of the types [Cu(L)(phen)](ClO4) 1-2, where HL is a tridentate ligand with two nitrogen and one oxygen donor atoms (2NO) such as 2-(2-(1H-benzimidazol-2-yl)ethyliminomethyl)phenol (HL1) and 2-(2-(1H-benzimidazol-2-yl)ethyl-imi... |
|
|
Helical-chain copper(II) complexes and a cyclic tetranuclear copper(II) complex with single syn-anti carboxylate bridges and ferromagnetic exchange interactions.
Inorg. Chem. 39(13) , 2882-90, (2000) Tridentate Schiff-base carboxylate-containing ligands, derived from the condensation of 2-imidazolecarboxaldehyde with the amino acids beta-alanine (H2L1) and 2-aminobenzoic acid (H2L5) and the condensation of 2-pyridinecarboxaldehyde with beta-alanine (HL2),... |
|
|
Enantioselective imidazole-directed allylation of aldimines and ketimines.
Org. Lett. 9 , 3699, (2007) A new chiral allylchlorosilane has been developed that allows the highly enantioselective allylation and crotylation of a range of 2-imidazolylaldimines and ketimines. The method may be exploited for the protecting group-free synthesis of a diverse array of i... |