docetaxel trihydrate
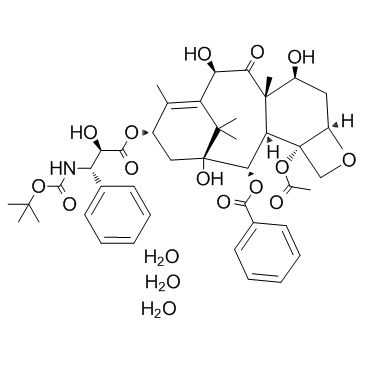
docetaxel trihydrate structure
|
Common Name | docetaxel trihydrate | ||
|---|---|---|---|---|
| CAS Number | 148408-66-6 | Molecular Weight | 861.925 | |
| Density | 1.37 g/cm3 | Boiling Point | 1016.9ºC at 760 mmHg | |
| Molecular Formula | C43H59NO17 | Melting Point | 186-192ºC | |
| MSDS | N/A | Flash Point | 568.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Acute lung injury associated with docetaxel and bevacizumab.
Clin. Oncol. (R. Coll. Radiol.) 19(10) , 803-5, (2007)
|
|
|
Second-line treatment of non-small cell lung cancer: big targets, small progress; small targets, big progress?
J. Thorac. Oncol. 1(9) , 927-8, (2006)
|
|
|
Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study.
Eur. Urol. 54(5) , 1089-94, (2008) Although the taxanes represent the most active agents for the first-line treatment of metastatic hormone-refractory prostate cancer (HRPC), most patients eventually progress while receiving taxane-based treatments. No agents are approved for second-line thera... |
|
|
Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer.
J. Clin. Oncol. 28(20) , 3239-47, (2010) The efficacy and safety of combining bevacizumab (7.5 and 15 mg/kg) with docetaxel as first-line therapy for human epidermal growth factor receptor 2 (HER2) -negative, locally recurrent or metastatic breast cancer (MBC) was investigated in a three-arm, placeb... |
|
|
Scheduling of taxanes: a review.
Curr. Clin. Pharmacol. 5(3) , 226-31, (2010) The taxanes are widely used in the cytotoxic treatment of many solid tumours. Their optimal scheduling, however, remains unconfirmed. Here we review the development of both paclitaxel and docetaxel to identify evidence influencing the choice of schedule. Earl... |
|
|
Editorial comment on: combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study.
Eur. Urol. 54(5) , 1094-6, (2008)
|
|
|
[Promising new treatment options for metastatic androgen-independent prostate cancer].
Actas Urol. Esp. 31(6) , 680-5, (2007) Review the recent advances in the treatment of androgen independent prostate cancer (AIPC).Review recent abstracts and literature utilizing Medline/PubMed using key words: androgen independent/hormone refractory prostate cancer, novel treatment options, Phase... |
|
|
Short-term health-related quality of life and symptom control with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF), 5-fluorouracil (PF) for induction in unresectable locoregionally advanced head and neck cancer patients (EORTC 24971/TAX 323).
Br. J. Cancer 103(8) , 1173-81, (2010) The EORTC 24971/TAX 323, a phase III study of 358 patients with unresectable locoregionally advanced squamous cell carcinoma of the head and neck, showed an improved progression-free and overall survival (OS) with less toxicity when docetaxel (T) was added to... |
|
|
Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer.
J. Clin. Oncol. 28(12) , 2070-6, (2010) PURPOSE We previously demonstrated that thalidomide appears to add to the activity of docetaxel in metastatic castration-resistant prostate cancer (CRPC). Phase II studies combining docetaxel with bevacizumab have had substantial antitumor activity. We hypoth... |
|
|
Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy.
Ann. Oncol. 21(9) , 1765-71, (2010) We investigated whether circulating tumor cells (CTCs) and circulating endothelial cells (CECs) predict clinical outcome of first-line chemotherapy combined with bevacizumab in metastatic breast cancer patients.In a French substudy of the MO19391 trial, CTC a... |