2-Methyl-L-serine
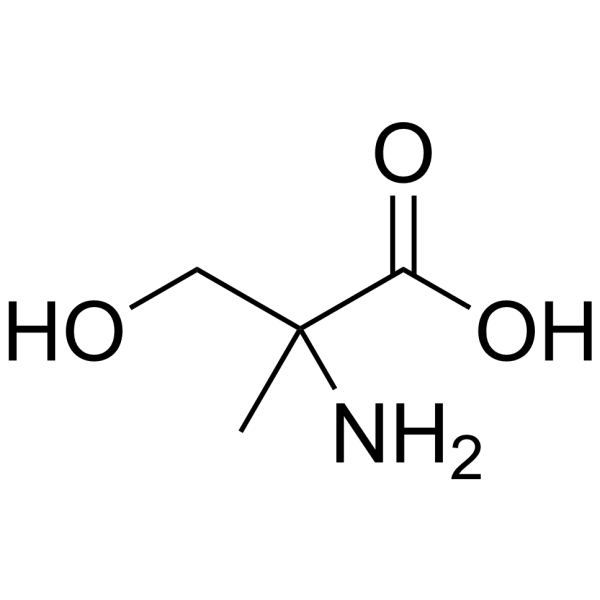
2-Methyl-L-serine structure
|
Common Name | 2-Methyl-L-serine | ||
|---|---|---|---|---|
| CAS Number | 5424-29-3 | Molecular Weight | 119.119 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 345.8±32.0 °C at 760 mmHg | |
| Molecular Formula | C4H9NO3 | Melting Point | 253-263ºC | |
| MSDS | USA | Flash Point | 162.9±25.1 °C | |
|
Drug design, in vitro pharmacology, and structure-activity relationships of 3-acylamino-2-aminopropionic acid derivatives, a novel class of partial agonists at the glycine site on the N-methyl-D-aspartate (NMDA) receptor complex.
J. Med. Chem. 52 , 5093-107, (2009) Retaining agonistic activity at the glycine coagonist site of the NMDA receptor in molecules derived from glycine or d-serine has proven to be difficult because in the vicinity of the alpha-amino acid group little substitution is tolerated. We have solved thi... |
|
|
The requirement of phosphorylation on a threonine residue in the acute regulation of steroidogenesis in MA-10 mouse Leydig cells.
J. Steroid Biochem. Mol. Biol. 46(3) , 337-47, (1993) In the present study we have used several non-phosphorylatable analogs of the amino acids threonine and serine to determine the role of phosphorylation in the acute regulation of steroidogenesis in MA-10 mouse Leydig tumor cells. Our results indicate that sub... |
|
|
Control of chemoselectivity in Dieckmann ring closures leading to tetramic acids.
Org. Biomol. Chem. 9 , 6663, (2011) An efficient strategy for the control of the chemoselectivity in Dieckmann ring closures leading to tetramic acids derived from serine and α-methyl serine is reported, and this provides pathways to diversely substituted systems from a common starting material... |
|
|
Synthesis, NMR spectra and function of peptides with alpha-methylserine attached to the RGD sequence of osteopontin.
Acta Chem. Scand. 46(10) , 989-93, (1992) The three-dimensional structure of the RGD-adhesion sequence has been studied previously by means of linear and cyclic peptides. These peptides show widely differing affinities to integrins, ascribed to a strong dependence on steric factors in the receptor re... |
|
|
Conformational studies on host-guest peptides containing chiral alpha-methyl-alpha-amino acids. Comparison of the helix-inducing potential of alpha-aminoisobutyric acid, (S)-2-ethylalanine and (S)-2-methylserine.
Int. J. Pept. Protein Res. 32(5) , 344-51, (1988) The conformational behaviour of host-guest peptides of the type Ac-Ala-Xxx-Ala-Ala-Xxx-Ala-Ala-Xxx-Ala-Ala-NH-PEGM (Xxx = alpha-aminoisobutyric acid (Aib), (S)-2-ethylalanine ((S)-Iva), (S)-2-methylserine ((S)-alpha-MeSer)) has been studied by CD spectroscopy... |
|
|
An approach to enantioselective activation of N-benzoyl-alpha-methylserine with chiral N-triazinylammonium chloride.
Acta Pol. Pharm. 63(5) , 426-9, (2006)
|
|
|
A strategy for glycine encephalopathy therapy.
J. Ment. Defic. Res. 26 (Pt 2) , 107-10, (1982) An inherited defect in the glycine cleavage enzyme results in the condition of neonatal glycine encephalopathy which has not responded to the current innovative methods of therapy. A re-examination of the enzyme structure and metabolic pathways, leads us to r... |
|
|
Conformational effects of the non-natural alpha-methylserine on small peptides and glycopeptides.
J. Org. Chem. 74(24) , 9305-13, (2009) The synthesis and the conformational analysis in aqueous solution of a peptide and a glycopeptide containing the sequence threonine-alanine-alanine (Thr-Ala-Ala) are reported. Furthermore, the threonine residue has been replaced by the quaternary amino acid a... |
|
|
Rational design of a Tn antigen mimic.
Chem. Commun. (Camb.) 47(18) , 5319-21, (2011) A novel Tn antigen mimic, in which the natural underlying amino acid has been replaced by the non-natural α-methylserine analogue, is reported. This derivative exhibits a similar affinity for a natural lectin as for the natural Tn and retains the bioactive co... |
|
|
Introduction of alpha-hydroxymethyamino acid residues in substrate specificity P1 position of trypsin inhibitor SFTI-1 from sunflower seeds retains its activity.
Biochem. Biophys. Res. Commun. 340(3) , 823-8, (2006) In many complexes formed by serine proteinases and their inhibitors, the hydroxyl group provided by water molecule or by the inhibitor Ser residue is located close to the inhibitor P1-P1' reactive site. In order to investigate the role of this group, we synth... |