2-Methyl-L-serine
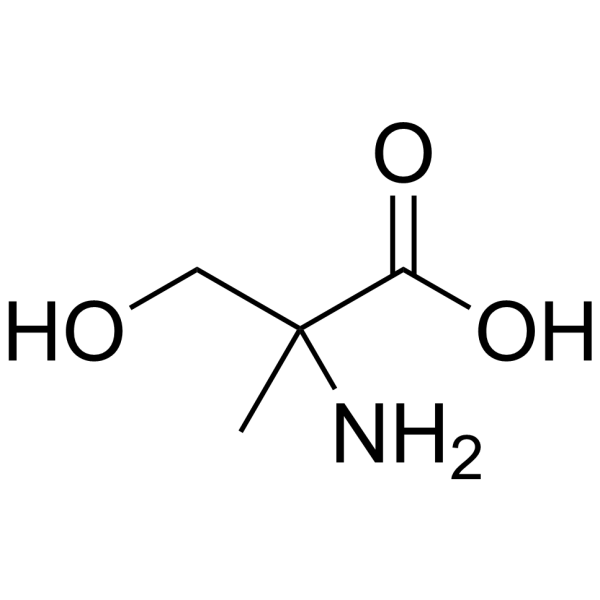
2-Methyl-L-serine structure
|
Common Name | 2-Methyl-L-serine | ||
|---|---|---|---|---|
| CAS Number | 5424-29-3 | Molecular Weight | 119.119 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 345.8±32.0 °C at 760 mmHg | |
| Molecular Formula | C4H9NO3 | Melting Point | 253-263ºC | |
| MSDS | USA | Flash Point | 162.9±25.1 °C | |
Use of 2-Methyl-L-serine2-Amino-3-hydroxy-2-methylpropanoic acid is a serine derivative[1]. |
| Name | .α.-Methyl-DL-serine |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Amino-3-hydroxy-2-methylpropanoic acid is a serine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 345.8±32.0 °C at 760 mmHg |
| Melting Point | 253-263ºC |
| Molecular Formula | C4H9NO3 |
| Molecular Weight | 119.119 |
| Flash Point | 162.9±25.1 °C |
| Exact Mass | 119.058243 |
| PSA | 83.55000 |
| LogP | -1.23 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.513 |
| InChIKey | CDUUKBXTEOFITR-UHFFFAOYSA-N |
| SMILES | CC(N)(CO)C(=O)O |
| Precursor 8 | |
|---|---|
| DownStream 3 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Drug design, in vitro pharmacology, and structure-activity relationships of 3-acylamino-2-aminopropionic acid derivatives, a novel class of partial agonists at the glycine site on the N-methyl-D-aspartate (NMDA) receptor complex.
J. Med. Chem. 52 , 5093-107, (2009) Retaining agonistic activity at the glycine coagonist site of the NMDA receptor in molecules derived from glycine or d-serine has proven to be difficult because in the vicinity of the alpha-amino acid... |
|
|
The requirement of phosphorylation on a threonine residue in the acute regulation of steroidogenesis in MA-10 mouse Leydig cells.
J. Steroid Biochem. Mol. Biol. 46(3) , 337-47, (1993) In the present study we have used several non-phosphorylatable analogs of the amino acids threonine and serine to determine the role of phosphorylation in the acute regulation of steroidogenesis in MA... |
|
|
Control of chemoselectivity in Dieckmann ring closures leading to tetramic acids.
Org. Biomol. Chem. 9 , 6663, (2011) An efficient strategy for the control of the chemoselectivity in Dieckmann ring closures leading to tetramic acids derived from serine and α-methyl serine is reported, and this provides pathways to di... |
| Serine,2-methyl-,DL |
| 2-methylserine |
| Serine, 2-methyl-, DL- |
| DL-A-METHYLSERINE |
| Dl-serine,2-methyl |
| 2-methyl-DL-serine |
| DL-Serine, 2-methyl- |
| A-METHYL-D,L-SERINE |
| L-Serine, 2-methyl- |
| DL-2-Methylserine |
| DL-2-Me-Ser-OH |
| 2-Methyl-L-serine |
| Alpha-Methyl-D,L-serine |
 CAS#:115-69-5
CAS#:115-69-5 CAS#:113845-95-7
CAS#:113845-95-7 CAS#:592-20-1
CAS#:592-20-1 CAS#:151-50-8
CAS#:151-50-8 CAS#:116-09-6
CAS#:116-09-6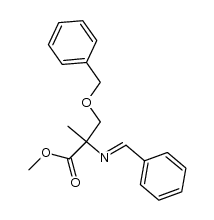 CAS#:120020-03-3
CAS#:120020-03-3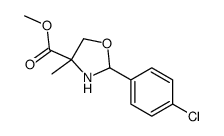 CAS#:109918-42-5
CAS#:109918-42-5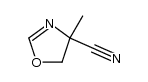 CAS#:58004-57-2
CAS#:58004-57-2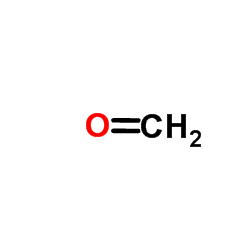 CAS#:50-00-0
CAS#:50-00-0 CAS#:302-72-7
CAS#:302-72-7 CAS#:22059-21-8
CAS#:22059-21-8
