Uridine diphosphate glucuronic acid ammonium
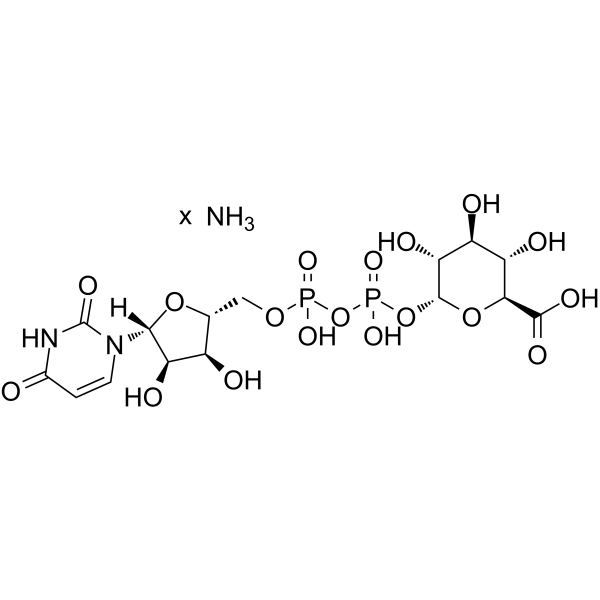
Uridine diphosphate glucuronic acid ammonium structure
|
Common Name | Uridine diphosphate glucuronic acid ammonium | ||
|---|---|---|---|---|
| CAS Number | 43195-60-4 | Molecular Weight | 597.31600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H25N3O18P2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Metabolic study of androsta-1,4,6-triene-3,17-dione in horses using liquid chromatography/high resolution mass spectrometry.
J. Steroid Biochem. Mol. Biol. 152 , 142-54, (2015) Androsta-1,4,6-triene-3,17-dione (ATD) is an irreversible steroidal aromatase inhibitor and is marketed as a supplement. It has been reported to effectively reduce estrogen biosynthesis and significantly increase the levels of endogenous steroids such as dihy... |
|
|
Stable isotope labeling strategy for curcumin metabolite study in human liver microsomes by liquid chromatography-tandem mass spectrometry.
J. Am. Soc. Mass Spectrom. 26(4) , 686-94, (2015) The identification of drug metabolites is very important in drug development. Nowadays, the most widely used methods are isotopes and mass spectrometry. However, the commercial isotopic labeled reagents are usually very expensive, and the rapid and convenient... |
|
|
Identification of diet-derived constituents as potent inhibitors of intestinal glucuronidation.
Drug Metab. Dispos. 42(10) , 1675-83, (2014) Drug-metabolizing enzymes within enterocytes constitute a key barrier to xenobiotic entry into the systemic circulation. Furanocoumarins in grapefruit juice are cornerstone examples of diet-derived xenobiotics that perpetrate interactions with drugs via mecha... |
|
|
Inhibition screening method of microsomal UGTs using the cocktail approach.
Eur. J. Pharm. Sci. 71 , 35-45, (2015) A rapid method for the simultaneous determination of the in vitro activity of the 10 major human liver UDP-glucuronosyltransferase (UGT) enzymes was developed based on the cocktail approach. Specific substrates were first selected for each UGT: etoposide for ... |
|
|
In vitro and in vivo biotransformation of WMS-1410, a potent GluN2B selective NMDA receptor antagonist.
J. Pharm. Biomed. Anal. 94 , 36-44, (2014) Structural modification of the GluN2B selective NMDA receptor antagonist ifenprodil led to the 3-benzazepine WMS-1410 with similar GluN2B affinity but higher receptor selectivity. Herein the in vitro and in vivo biotransformation of WMS-1410 is reported. Incu... |
|
|
Identification of saturated and unsaturated fatty acids released during microsomal incubations.
Xenobiotica 44(8) , 687-95, (2014) 1. In vitro clearance in liver microsomes is routinely measured in drug discovery and development for new chemical entities. Literature reports indicate that long chain fatty acids such as arachidonic, linoleic and oleic acids may be released over a period of... |
|
|
Pharmacokinetics, tissue distribution, and tentative metabolite identification of sauchinone in mice by microsampling and HPLC-MS/MS methods.
Biol. Pharm. Bull. 38(2) , 218-27, (2015) Sauchinone, a biologically active lignan found in Saururus chinensis (Saururaceae), exerts various biological activities against jaundice, inflammatory disease, hepatic steatosis, and oxidative injury. Despite its diverse applications, there exists some infor... |
|
|
In vitro glucuronidation of five rhubarb anthraquinones by intestinal and liver microsomes from humans and rats.
Chem. Biol. Interact. 219 , 18-27, (2014) Anthraquinones naturally distribute in many plants including rhubarb and have widespread applications throughout industry and medicine. Recent studies provided new insights in potential applications of these traditional laxative constituents. Glucuronidation ... |
|
|
Regioselective glucuronidation of oxyresveratrol, a natural hydroxystilbene, by human liver and intestinal microsomes and recombinant UGTs.
Drug Metab. Pharmacokinet. 29(3) , 229-36, (2014) Oxyresveratrol (OXY) is a natural hydroxystilbene that shows similar bioactivity but better water solubility than resveratrol. This study aims to characterize its glucuronidation kinetics in human liver (HLMs) and intestinal (HIMs) microsomes and identify the... |
|
|
Metabolic drug-drug interaction potential of macrolactin A and 7-O-succinyl macrolactin A assessed by evaluating cytochrome P450 inhibition and induction and UDP-glucuronosyltransferase inhibition in vitro.
Antimicrob. Agents Chemother. 58(9) , 5036-46, (2014) Macrolactin A (MA) and 7-O-succinyl macrolactin A (SMA), polyene macrolides containing a 24-membered lactone ring, show antibiotic effects superior to those of teicoplanin against vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureu... |