Pinane
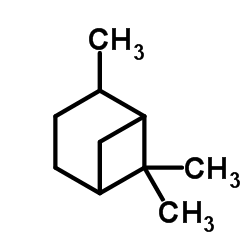
Pinane structure
|
Common Name | Pinane | ||
|---|---|---|---|---|
| CAS Number | 4795-86-2 | Molecular Weight | 138.250 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 157.0±7.0 °C at 760 mmHg | |
| Molecular Formula | C10H18 | Melting Point | -53ºC | |
| MSDS | USA | Flash Point | 36.0±11.7 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
|
Metabolic profiling of GuanXin II prescription based on metabolic fingerprinting and chemical analysis.
J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011) A sensitive LC/MS method was established to investigate the in vivo metabolism of GuanXin II prescription, a five-component Chinese herbal medicine formulation. Rat plasma, bile, urine, and feces were collected and analyzed following oral administration of th... |
|
|
Chemistry around pinene and pinane: a facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from cis- and trans-pinanols.
Chem. Biodivers. 5(6) , 910-9, (2008) Alpha-pinene and beta-pinene are abundantly represented in nature; they are obtained from renewable sources and are irreplaceable synthons for commercial-scale production of other terpenes. In a well-known process practiced by Millennium Specialty Chemicals (... |
|
|
Acute toxicities to larval rainbow trout of representative compounds detected in Great Lakes fish.
Bull. Environ. Contam. Toxicol. 46(2) , 173-8, (1991)
|
|
|
Role of thromboxane and angiotensin in cyclosporine-induced renal vasoconstriction in the dog.
J. Heart Lung Transplant. 12(5) , 851-5, (1993) Cyclosporine is associated with renal insufficiency characterized by a reduction in glomerular filtration rate that may result from renal vasoconstriction. Injection of cyclosporine in the isolated renal artery perfused at a constant flow induces a potent dos... |
|
|
Selective boron-containing thrombin inhibitors--X-ray analysis reveals surprising binding mode.
Bioorg. Med. Chem. 8(9) , 2291-303, (2000) Based on the structural comparison of the S1 pocket in different trypsin-like serine proteases, a series of Boc-D-trimethylsilylalanine-proline-boro-X pinanediol derivatives, with boro-X being different amino boronic acids, have been synthesized as inhibitors... |
|
|
Terpenoids biotransformation in mammals III: Biotransformation of alpha-pinene, beta-pinene, pinane, 3-carene, carane, myrcene, and p-cymene in rabbits.
J. Pharm. Sci. 70(4) , 406-15, (1981) The biotransformation of (+)-, (-)-, and (+-)-alpha-pinenes, (-)-beta-pinene (nopinene), (-)-cis-pinane, (+)-3-carene, (-)-cis-carane, myrcene, and p-cymene in rabbits was investigated. The major metabolites were as follows: (-)-trans-verbenol from (+)-, (-)-... |
|
|
Monoterpene-based chiral β-amino acid derivatives prepared from natural sources: syntheses and applications.
Amino Acids 41(3) , 597-608, (2011) Natural monoterpenes have proved to be good starting materials for the synthesis of β-amino acid derivatives. In the past decade, a number of well-known synthetic procedures have been applied for the preparation of monoterpene-based β-amino acid derivatives, ... |
|
|
Synthesis of mesoporous silica with embedded nickel nanoparticles for catalyst applications.
J. Nanosci. Nanotechnol. 2(1) , 89-94, (2002) Here we describe a new route for the synthesis of nanometric Ni particles embedded in a mesoporous silica material with excellent potential for catalytic applications. Mesoporous silica with a surface area in the range of 202-280 m2/g, with narrow pore size d... |
|
|
Thermal isomerization of (+)-cis- and (-)-trans-pinane leading to (-)-beta-citronellene and (+)-isocitronellene.
Chemistry 14(22) , 6805-14, (2008) Catalyzed and uncatalyzed rearrangement reactions of terpenoids play a major role in laboratory and industrial-scale synthesis of fine chemicals. Herein, we present our results on the thermally induced isomerization of pinane (1). Investigation of the thermal... |
|
|
Sublethal effects of phenanthrene, nicotine, and pinane on Daphnia pulex.
Bull. Environ. Contam. Toxicol. 42(5) , 778-84, (1989)
|