1,2-Ethanediamine,N1,N2-diphenyl
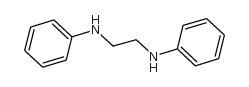
1,2-Ethanediamine,N1,N2-diphenyl structure
|
Common Name | 1,2-Ethanediamine,N1,N2-diphenyl | ||
|---|---|---|---|---|
| CAS Number | 150-61-8 | Molecular Weight | 212.29000 | |
| Density | 1.121g/cm3 | Boiling Point | 228°C 12mm | |
| Molecular Formula | C14H16N2 | Melting Point | 65-67 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 228°C/12mm | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Role of dermal exposure in systemic intake of methylenediphenyl diisocyanate (MDI) among construction and boat building workers.
Toxicol. Lett. 232(3) , 595-600, (2015) The causal relationship between inhalation exposure to methylenediphenyl diisocyanate (MDI) and the risk of occupational asthma is well known, but the role of dermal exposure and dermal uptake of MDI in this process is still unclear. The aims of this study we... |
|
|
Reversible and irreversible interactions of a cisplatin analog bearing a 1,2-diphenylethylenediamine ligand with plasma and plasma proteins in vitro.
Drug Metab. Dispos. 22(3) , 419-27, (1994) The cisplatin analog [meso-1,2-bis(2,6-dichloro-4-hydroxyphenyl) ethylenediamine]dichloroplatinum(II) [PtCl2(1)], by virtue of its estrogenic 1,2-diphenylethylenediamine ligand 1, was intended to function as a cytotoxic estrogen. This article reports on the r... |
|
|
Preparation and enantioseparation of a mixed selector chiral stationary phase derived from benzoylated tartaric acid and 1,2-diphenylethylenediamine.
Chirality 22(6) , 604-11, (2010) L-Dibenzoyl tartaric acid was mono-esterified with benzyl alcohol, and then chlorinated with SOCl(2) to give (2S,3S)-1-(benzyloxy)-4-chloro-1,4-dioxobutane-2,3-diyl dibenzoate (Selector 1). (1R,2R)-1,2-Diphenylethylenediamine was mono-functionalized with phen... |
|
|
Synthesis of dendritic stationary phases with surface-bonded L-phenylalanine derivate as chiral selector and their evaluation in HPLC resolution of racemic compounds.
Chirality 20(7) , 846-55, (2008) Four dendrimers were synthesized on aminopropyl-modified silica gel using methyl acrylate and ethylene diamine as building blocks by divergent method. Four generations of chiral stationary phases (CSPs) were prepared by coupling of L-2-(p-toluenesulfonamido)-... |
|
|
Synthesis of polymer-type chiral stationary phases and their enantioseparation evaluation by high-performance liquid chromatography.
Chirality 19(2) , 129-40, (2007) Two new chiral polymers of different molecular weights were synthesized by the copolymerization of (1R,2R)-(+)-1,2-diphenylethylenediamine, phenyl diisocyanate and terephthaloyl chloride. The polymers were immobilized on aminated silica gel to afford two chir... |
|
|
Spectrofluorometric determination of catechins with 1,2-diphenylethylenediamine.
Anal. Sci. 18(8) , 951-3, (2002)
|
|
|
High-performance liquid chromatographic determination of urinary catecholamines by direct pre-column fluorescence derivatization with 1,2-diphenylethylenediamine.
J. Chromatogr. A. 380(1) , 229-31, (1986)
|
|
|
Simultaneous determination of 5-hydroxyindoles and catechols by high-performance liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine.
J. Chromatogr. A. 1012(2) , 169-77, (2003) A highly selective and sensitive method for the simultaneous determination of 5-hydroxyindoles and catechols (serotonin, norepinephrine, dopamine and related compounds) by high-performance liquid chromatography with fluorescence detection is described. The me... |
|
|
Determination of serotonin, noradrenaline, dopamine and their metabolites in rat brain extracts and microdialysis samples by column liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 807(2) , 177-83, (2004) A highly selective and sensitive column liquid chromatographic method for fluorescence determination of serotonin (5-HT), dopamine (DA), noradrenaline (NA) and their related metabolites 5-hydroxyindole-3-acetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid... |
|
|
Successive optical resolution of two compounds by one enantiopure compound.
Chem. Commun. (Camb.) (10) , 1070-2, (2006) By using (1R,2R)-1,2-diphenylethylenediamine as a single enantiopure compound, we achieved a novel successive optical resolution of more than one kind of racemic compound through supramolecular crystallization. |