1-acetoxy-1,3-butadiene
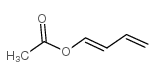
1-acetoxy-1,3-butadiene structure
|
Common Name | 1-acetoxy-1,3-butadiene | ||
|---|---|---|---|---|
| CAS Number | 1515-76-0 | Molecular Weight | 112.12700 | |
| Density | 0.96 g/mL at 20 °C(lit.) | Boiling Point | 60-61 °C40 mm Hg(lit.) | |
| Molecular Formula | C6H8O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 92 °F | |
| Symbol |


GHS02, GHS06 |
Signal Word | Danger | |
|
J. Chem. Soc. Perkin Trans. I , 1925, (1993)
|
|
|
Preparation of functionalized juglone acetates and juglones via 1, 4-dimethoxynaphthalene derivatives: synthesis of anthraquinones related to rhein and aloe-emodin. Bloomer JL, et al.
J. Org. Chem. 58(27) , 7906, (1993)
|
|
|
J. Org. Chem. 58 , 7146, (1993)
|
|
|
Intra- and Intermolecular Oxa-Pictet-Spengler Cyclization Strategy for the Enantioselective Synthesis of Deoxy Analogues of (+)-Nanomycin A Methyl Ester, (+)-Eleutherin, (+)-Allo-Eleutherin, and (+)-Thysanone R. T. Sawant, et al.
European J. Org. Chem. 23 , 4442-4449, (2010)
|
|
|
Structure determination of the Diels-Alder product of a ketovinylphosphonate with< i> E</i>-1-acetoxy-1, 3-butadiene. McClure CK, et al.
Tetrahedron Lett. 37(13) , 2153-6, (1996)
|
|
|
Enantio-face control by molecular sieves in the asymmetric Diels-Alder reaction Moharram SM, et al.
Tetrahedron Lett. 41(34) , 6669-73, (2000)
|
|
|
Cis and trans 1-acetoxy-1, 3-butadiene: physical and chemical properties, infrared and ultraviolet spectra. Georgieff KK and Dupré A.
Can. J. Chem. 38(7) , 1070-1075, (1960)
|
|
|
Gas phase acetoxylation of 1, 3-butadiene over palladium catalysts Part 1: the catalytic activity and structure of Pd-Sb-KOAc catalysts. Shinohara H.
Appl. Catal. 10(1) , 27-42, (1984)
|
|
|
Radical cation Diels-Alder cycloadditions by visible light photocatalysis.
J. Am. Chem. Soc. 133 , 19350-19353, (2011) Ruthenium(II) polypyridyl complexes promote the efficient radical cation Diels-Alder cycloaddition of electron-rich dienophiles upon irradiation with visible light. These reactions enable facile [4 + 2] cycloadditions that would be electronically mismatched u... |
|
|
Synthesis and Diels-Alder reactivity of ortho-carbazolequinones.
Chem. Pharm. Bull. 52(9) , 1114-6, (2004) Oxidation of 2- and 3-hydroxycarbazoles with Frémy's salt gave the corresponding ortho-carbazolequinones. These molecules react as carbodienophiles in Diels-Alder reaction with 1-acetoxy-1,3-butadiene and 1,3-cyclopentadiene to provide the novel benzocarbazol... |