H-Phe-ol
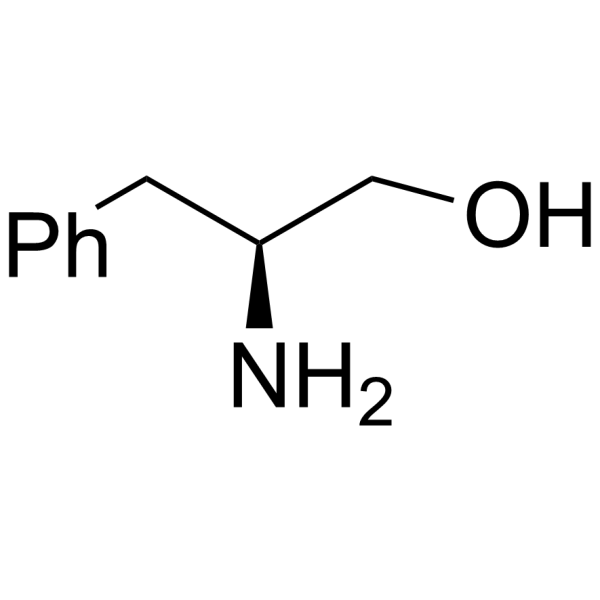
H-Phe-ol structure
|
Common Name | H-Phe-ol | ||
|---|---|---|---|---|
| CAS Number | 3182-95-4 | Molecular Weight | 151.206 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 303.8±22.0 °C at 760 mmHg | |
| Molecular Formula | C9H13NO | Melting Point | 92-94ºC | |
| MSDS | Chinese USA | Flash Point | 137.5±22.3 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Straightforward methodology for the enantioselective synthesis of benzo[a]- and indolo[2,3-a]quinolizidines.
J. Org. Chem. 72(14) , 5193-201, (2007) An enantioselective two-step route to substituted benzo[a]- and indolo[2,3-a]quinolizidines has been developed. It consists of (i) a stereoselective cyclocondensation of a racemic or prochiral delta-oxo(di)ester with either (S)-(3,4-dimethoxyphenyl)alaninol o... |
|
|
Direct high-performance liquid chromatographic separation of the enantiomers of an aromatic amine and four aminoalcohols using polysaccharide chiral stationary phases and acidic additive.
Chirality 19(8) , 647-53, (2007) The HPLC enantiomeric separation of N-benzyl-alpha-methyl-benzylamine, phenylalaninol, tryptophanol, 2 (diphenylhydroxymethyl)pyrrolidine, and isoproterenol was accomplished in the normal-phase mode using two polysaccharide-derived chiral stationary phases (C... |
|
|
Paracelsin; characterization by NMR spectroscopy and circular dichroism, and hemolytic properties of a peptaibol antibiotic from the cellulolytically active mold Trichoderma reesei. Part B.
Experientia 40 , 1189, (1984) Paracelsin, a hemolytic and membrane active polypeptide antibiotic of the peptaibol class which is excreted by the mold Trichoderma reesei, was obtained by a simplified and rapid isolation procedure utilizing hydrophobic adsorber resins. Investigation by 13C ... |
|
|
Relationship between membrane fluidity and capping of receptors for concanavalin A.
Can. J. Biochem. 58(12) , 1421-9, (1980) The activities of a range of phenylalaninol-related compounds on capping of concanavalin A and induction of rounding of Chinese hamster ovary tsHl cells, as well as on the fluidity of phosphatidylcholine-cholesterol (1:1) liposomes, have been examined. These ... |
|
|
Enhanced sensitivity of tumorigenic cells to rapid rounding induced by phenylalaninol.
Can. J. Biochem. 58(9) , 737-44, (1980) This study describes a rounding reaction induced in mammalian cells by the addition of phenylalaninol. In the Chinese hamster ovary tsH1 line the rounding occurred rapidly with a half time of 1 min at 25 mM phenylalaninol. After the removal of phenylalaninol,... |
|
|
Chiral bis(amino alcohol)oxalamide gelators-gelation properties and supramolecular organization: racemate versus pure enantiomer gelation.
Chemistry 9(22) , 5567-80, (2003) Four new chiral bis(amino alcohol)oxalamides (1-4: amino alcohol=leucinol, valinol, phenylglycinol, and phenylalaninol, respectively) have been prepared as low-molecular-weight organic gelators. Their gelation properties towards various organic solvents and m... |
|
|
tRNAPhe and tRNAPro are the near-ultraviolet molecular targets triggering the growth delay effect.
Biochem. Biophys. Res. Commun. 150(3) , 979-86, (1988) The illumination of Escherichia coli cells with UVA light, 320 nm less than or equal to lambda less than or equal to 380 nm, triggers a transient growth and division delay. The built-in 4-thiouridine chromophore which absorbs light at 340 nm leads to the quan... |
|
|
A colorimetric chiral sensor based on chiral crown ether for the recognition of the two enantiomers of primary amino alcohols and amines.
Chirality 23(4) , 349-53, (2011) A new colorimetric chiral sensor material consisting of three different functional sites such as chromophore (2,4-dinitrophenylazophenol dye), binding site (crown ether), and chiral barrier (3,3'-diphenyl-1,1'-binaphthyl group) was prepared and applied to the... |
|
|
Aminothiols: synthesis and effect on chicken brain aminopeptidases.
Res. Commun. Chem. Pathol. Pharmacol. 62(1) , 113-23, (1988) An amino acid derivative, leucinethiol, was reported to be a strong inhibitor of aminopeptidase activity. In order to obtain selective inhibitors of various brain aminopeptidases, we tested the inhibition by amino acid analogs of brain aminopeptidase activity... |
|
|
Synthesis and activity of 2-oxoamides containing long chain beta-amino acids.
J. Pept. Sci. 11(7) , 431-5, (2005) 2-Oxoamides based on long chain beta-amino acids were synthesized. 1-Benzyl substituted long chain amines, needed for such synthesis, were synthesized starting from Boc-phenylalaninol. The oxidative conversion of a phenyl group to a carboxyl group was used as... |