2-Iodothiophene
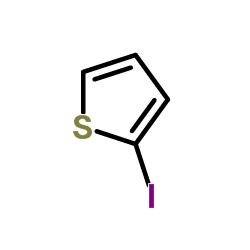
2-Iodothiophene structure
|
Common Name | 2-Iodothiophene | ||
|---|---|---|---|---|
| CAS Number | 3437-95-4 | Molecular Weight | 210.036 | |
| Density | 2.1±0.1 g/cm3 | Boiling Point | 181.8±13.0 °C at 760 mmHg | |
| Molecular Formula | C4H3IS | Melting Point | −40 °C(lit.) | |
| MSDS | USA | Flash Point | 71.1±0.0 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Rapid homogeneous-phase Sonogashira coupling reactions using controlled microwave heating.
J. Org. Chem. 66(12) , 4165-9, (2001) A microwave-enhanced, rapid and efficient homogeneous-phase version of the Sonogashira reaction is presented. It has been applied to the coupling of aryl iodides, bromides, triflates, and aryl chloride, as well as pyridine and thiophene derivatives with trime... |
|
|
Cross-coupling reactions of alkenylsilanolates. Investigation of the mechanism and identification of key intermediates through kinetic analysis.
J. Am. Chem. Soc. 126(15) , 4876-82, (2004) The mechanism of the fluoride-free, palladium-catalyzed cross-coupling reaction of potassium (E)-heptenyldimethylsilanolate, K(+)(E)-1(-), with 2-iodothiophene has been investigated through kinetic analysis. The order of each component was determined by plott... |
|
|
Stereospecific preparation of (Z)- and (E)-2,3-difluoro-3-stannylacrylic ester synthons and a new, efficient stereospecific route to (Z)- and (E)-2,3-difluoroacrylic esters.
J. Org. Chem. 70(26) , 10743-6, (2005) [reaction: see text] The (Z)-2,3-difluoro-3-stannylacrylic ester is readily prepared from (Z)-1,2-difluorovinyltriethylsilane via stereospecific stannyl/silyl exchange with KF/(Bu3Sn)2O or Bu3SnCl in DMF at 70 degrees C. The corresponding (E)-2,3-difluoro-3-s... |
|
|
Near ultraviolet photochemistry of 2-bromo- and 2-iodothiophene: Revealing photoinduced ring opening in the gas phase?
J. Chem. Phys. 142 , 224303, (2015) Velocity map imaging methods, with a new and improved ion optics design, have been used to explore the near ultraviolet photodissociation dynamics of gas phase 2-bromo- and 2-iodothiophene molecules. In both cases, the ground (X) and spin-orbit excited (X*) (... |
|
|
Thin conductive coatings formed by plasma polymerization of 2-iodothiophene. Kruse A, et al.
Surf. Coat. Technol. 59(1) , 359-64, (1993)
|