Ethoxyethene
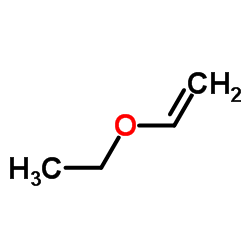
Ethoxyethene structure
|
Common Name | Ethoxyethene | ||
|---|---|---|---|---|
| CAS Number | 109-92-2 | Molecular Weight | 72.106 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 32.5±9.0 °C at 760 mmHg | |
| Molecular Formula | C4H8O | Melting Point | −116 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | -45.6±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
|
Unraveling the interplay of backbone rigidity and electron rich side-chains on electron transfer in peptides: the realization of tunable molecular wires.
J. Am. Chem. Soc. 136(35) , 12479-88, (2014) Electrochemical studies are reported on a series of peptides constrained into either a 310-helix (1-6) or β-strand (7-9) conformation, with variable numbers of electron rich alkene containing side chains. Peptides (1 and 2) and (7 and 8) are further constrain... |
|
|
Well-defined diblock brush polymer-drug conjugates for sustained delivery of paclitaxel.
Biomater. Sci. 3 , 1078-84, (2015) Using the 3(rd) generation Grubbs' catalyst as the initiator, diblock brush polymer drug conjugates (BPDCs) were synthesized by sequential ring-opening metathesis polymerization (ROMP) of a hydrophilic poly(ethylene glycol) (PEG)-based norbornene (NB)-functio... |
|
|
Influence of Alkoxy Groups on the Photoinduced Dynamics of Organic Molecules Exemplified on Alkyl Vinyl Ethers.
J. Phys. Chem. A 119 , 11105-12, (2015) A series of different alkyl vinyl ethers is investigated to decipher the possible reaction channels upon photoexcitation to the π3s-Rydberg and the ππ*-valence state at 200 nm using time-resolved photoelectron spectroscopy and on-the-fly time-dependent densit... |
|
|
Continuous-flow synthesis of monoarylated acetaldehydes using aryldiazonium salts.
J. Am. Chem. Soc. 134(30) , 12466-9, (2012) Anilines and ethyl vinyl ether can be used as precursors for a process that is the synthetic equivalent of the α-arylation of acetaldehyde enolate. The reaction manifests a high level of functional group compatibility, allowing the ready preparation of a numb... |
|
|
Au(I)-catalyzed efficient synthesis of functionalized bicyclo[3.2.0]heptanes.
J. Am. Chem. Soc. 130(22) , 6944-5, (2008) An efficient Au(I)-catalyzed synthesis of highly strained and functionalized bicyclo[3.2.0]heptanes is developed. Subsequent couplings with various nucleophiles offer additional structural features/complexity. These one-pot, three-component reactions are prop... |
|
|
Reactions of the peroxo intermediate of soluble methane monooxygenase hydroxylase with ethers.
J. Am. Chem. Soc. 127(20) , 7370-8, (2005) Soluble methane monooxygenase (sMMO) isolated from Methylococcus capsulatus (Bath) utilizes a carboxylate-bridged diiron center and dioxygen to catalyze the conversion of methane to methanol. Previous studies revealed that a di(mu-oxo)diiron(IV) intermediate ... |
|
|
Kinetic study of the gas-phase reactions of OH and NO3 radicals and O3 with selected vinyl ethers.
J. Phys. Chem. A 110(23) , 7386-92, (2006) Kinetic studies on the gas-phase reactions of OH and NO3 radicals and ozone with ethyl vinyl ether (EVE), propyl vinyl ether (PVE) and butyl vinyl ether (BVE) have been performed in a 405 L borosilicate glass chamber at 298 +/- 3 K in synthetic air using in s... |
|
|
Building addressable libraries: spatially isolated, chip-based reductive amination reactions.
J. Am. Chem. Soc. 128(1) , 70-1, (2006) A Pd(II) reagent has been generated at preselected sites on an electrochemically addressable chip and used to effect the oxidation of the neighboring alcohols on the polymer coating the chip's surface. The resulting carbonyls were then used to accomplish site... |
|
|
Identification of epoxide functionalities in protonated monofunctional analytes by using ion/molecule reactions and collision-activated dissociation in different ion trap tandem mass spectrometers.
J. Am. Soc. Mass Spectrom. 23(1) , 12-22, (2012) A mass spectrometric method has been delineated for the identification of the epoxide functionalities in unknown monofunctional analytes. This method utilizes gas-phase ion/molecule reactions of protonated analytes with neutral trimethyl borate (TMB) followed... |