4-ethvlpvridine
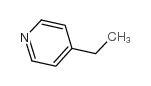
4-ethvlpvridine structure
|
Common Name | 4-ethvlpvridine | ||
|---|---|---|---|---|
| CAS Number | 536-75-4 | Molecular Weight | 107.15300 | |
| Density | 0.942 g/mL at 25 °C(lit.) | Boiling Point | 168 °C(lit.) | |
| Molecular Formula | C7H9N | Melting Point | -91 °C | |
| MSDS | Chinese USA | Flash Point | 118 °F | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
|
Evaluation of stationary phases packed with superficially porous particles for the analysis of pharmaceutical compounds using supercritical fluid chromatography.
J. Chromatogr. A. 1360 , 275-87, (2014) Superficially porous particles (SPP), or core shell particles, which consist of a non-porous silica core surrounded by a thin shell of porous silica, have gained popularity as a solid support for chromatography over the last decade. In the present study, five... |
|
|
Ambient preparation and reactions of gas phase silver cluster cations and anions.
Phys. Chem. Chem. Phys. 17 , 18364-73, (2015) Electrospray ionization of metal salt solutions followed by ambient heating transforms the resulting salt clusters into new species, primarily naked ionic metal clusters. The experiment is done by passing the clusters through a heated coiled loop outside the ... |
|
|
Isolation of oxidative degradation products of atorvastatin with supercritical fluid chromatography.
Biomed. Chromatogr. 29 , 1901-6, (2015) The isolation of four oxidative degradation products of atorvastatin using preparative high-performance liquid chromatography applying at least two chromatographic steps is known from the literature. In this paper it is shown that the same four impurities cou... |
|
|
Simple chip-based interfaces for on-line monitoring of supramolecular interactions by nano-ESI MS.
Lab Chip 5(10) , 1111-22, (2005) Two simple interfaces were designed and realized, enabling on-line coupling of microfluidics reactor chips to a nanoflow electrospray ionization (NESI) time-of-flight (TOF) mass spectrometer (MS). The interfaces are based on two different approaches: a monoli... |
|
|
Effect of counter-anion concentration on retention in high-performance liquid chromatography of protonated basic analytes.
J. Chromatogr. A. 913(1-2) , 189-96, (2001) The influence of acid and salt concentration in the mobile phase on the retention of basic analytes has been studied. An increase in the retention of fully protonated analytes with increasing the concentration of inorganic additives was found. The addition of... |